
 |
Self-discharge is the electrical capacity that is lost when the cell simply sits on the shelf. Self-discharge is caused by electrochemical processes within the cell and is equivalent to the application of a small external load. Li-ion cells typically lose 8% of their capacity for the first month and then 2% for subsequent months. In many cases the rate of loss may decline after a time due, for instance, to the build-up of a passivation film on lithium anodes. In order to reduce self-discharge, it is recommended to store cells and batteries at lower temperatures.
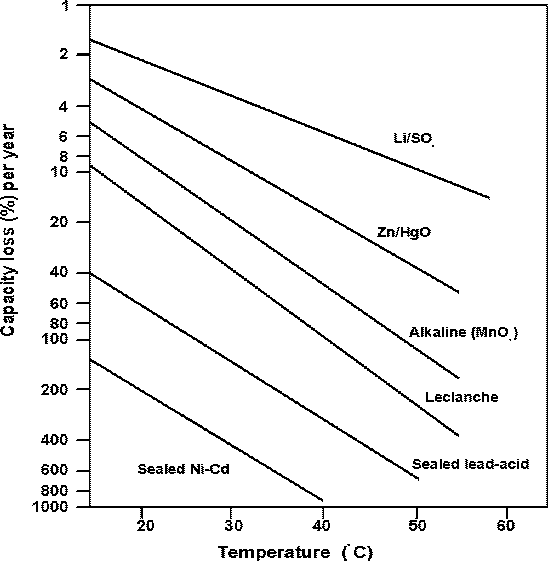
Typically Ni/Cd and Ni/MH cells suffer self-discharge rates as high as 25% per month. This presents the user with a major logistical problem since charging is normally always required before Ni/Cd batteries are used in the field.
Lead-acid and nickel-cadmium batteries lose their charge very quickly. For example, a lead-acid battery stored at 30oC would lose half its initial charge in about 3 or 4 months while, for nickel-cadmium, this would only take about 6 weeks. In normal use, this might present no real problem, as these types of battery can be recharged, but such batteries are clearly unsuitable for "fitting and forgetting".
Alkaline batteries hold their charge better than zinc-carbon unless they corrode badly as shown here, but lithium primary batteries have particularly good storage characteristics, due to the passivation layer that forms on the anode surface at open circuit. In most cases it is best to store batteries in a cool place and to warm them before use.