
 |
Magnesium has an equilibrium potential of -2.61V vs. SCE and therefore theoretically can provide a very large driving voltage . However, practical measurements indicate relatively more noble corrosion potentials probably due to the electrochemical inefficiency of the metal as a sacrificial anode. The low efficiency (50-60%) has been attributed to hydrogen evolution at local cathodes and complex surface chemistry at the anode surface. The theoretical current capacity for a magnesium anode is approximately 2200 Ah kg-1 whereas actual measured values are in the range of 1200 Ah kg-1.
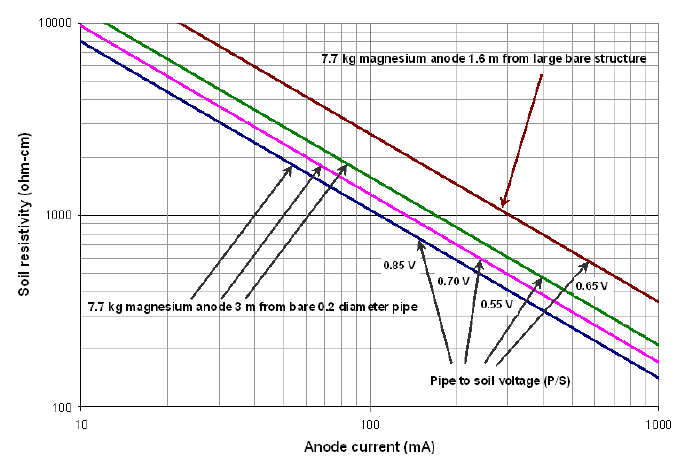
Typical performance curve for magnesium anodes (reference)
Alloying elements (Al, Zn, Mn) have been added to reduce the rapid activation of magnesium. Magnesium alloy anodes because of their large driving voltage are principally used in soils, water tanks, and similar high resistance media. In high conductivity environments, such as seawater, magnesium anodes are generally not recommended because of risk of overprotection and high consumption rate.