Galvanizing
Hot dip galvanizing is the process of applying a zinc coating to fabricated iron or steel material by immersing the material in a bath consisting primarily of molten zinc. The simplicity of the galvanizing process is a distinct advantage over other methods of providing corrosion protection. The automotive industry depends heavily on this process for the production of many components used in car manufacturing. Galvanizing forms a metallurgical bond between the zinc and the underlying steel or iron, creating a barrier that is part of the metal itself.
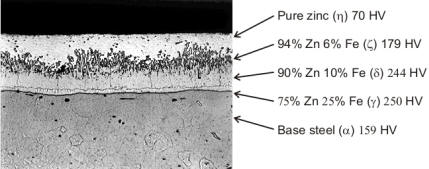
| During galvanizing, the molten zinc reacts with the iron in the steel to form a series of zinc/iron alloy layers. The photomicrograph of a galvanized steel coating cross-section shows a typical coating microstructure consisting of three alloy layers and a layer of pure metallic zinc. (HV stands for Vickers hardness) |
|
Service life of hot-dip galvanized coatings
Galvanizing can be found in almost every major application and industry where iron or mild steel is used. The utilities, chemical process, pulp and paper, automotive, and transportation industries, to name just a few, have historically made extensive use of galvanizing for corrosion control. The electrochemical protection provided to steel by zinc coatings is a vital element in the effectiveness of galvanized coatings in protecting steel from corrosion. All pre-galvanized products rely on the cathodic protection provided by zinc to prevent corrosion of exposed steel at cut edges.
While the potential difference between metals is the prime driving force providing the corrosion current, it is not a reliable guide to the rate and type of corrosion occurring at a particular point. The severity of galvanic corrosion also depends on the ratio of the areas of metals in contact, the duration of wetness (galvanic corrosion can only occur in the presence of a conductive solution) and the conductivity of the electrolyte. The presence of oxide films on the surface of one or both of the metals can greatly inhibit galvanic corrosion.
 |