 |
Metallic corrosion can produce very corrosive environments through the chemical change of water into acid called hydrolysis. This phenomenon is particularly noticeable when the environment is confined such as in most forms of localized corrosion (Pitting, crevice, environmental cracking). The chemical change in question is true of most metals since the metallic ions produced by the corrosion processes are not soluble in their ionic forms. These ions will then to react and form more stable species such as oxides and hydroxides as illustrated in the following example given for iron. In aerated environments iron oxidize to ferric ions that subsequently react with water:
(corrosion)Fe ® Fe2+
(further oxidation in air)Fe2+ ® Fe3+ + 1 e-
(hydrolysis)Fe3+ + 3 H2O ® Fe(OH)3 + 3 H+
At equilibrium the ratio between chemical concentrations involved in the hydrolysis (step 3) can be written as:

The activities of each species can be expressed in concentration units but since rust (Fe(OH)3) and water are pure species (activity of a pure species = 1) the equilibrium constant (Keq) simplifies to:
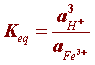
Since the acidity of an environment is expressed as:
![]()
It is possible to relate the pH of a corroding environment to the equilibrium of the metal exposed to this environment. The following numbers were produced with KTS Thermo as one easy output of the thermodynamics calculations. n in the fifth column indicates the number of protons for the molecule listed in column two.
Metal | Formula | Keq | log10(Keq) | n | pH |
| Aluminum (Al) | |||||
25°C | Al(OH)3 | 10-10.48 | -10.48 | 3 | 3.4 |
Al2O3.H2O | 10-16.37 | -16.37 | 6 | 2.7 | |
70°C | Al(OH)3 | 10-8.46 | -8.46 | 3 | 2.8 |
Al2O3.H2O | 10-11.14 | -11.14 | 6 | 1.8 | |
Chromium (Cr) | |||||
25°C | Cr(OH)3 | 10-4.49 | -4.49 | 3 | 1.4 |
CrOOH | 10-2.89 | -2.89 | 3 | 0.9 | |
70°C | Cr(OH)3 | 10-3.04 | -3.04 | 3 | 1.0 |
CrOOH | 10-1.53 | -1.53 | 3 | 0.5 | |
Iron or steel (Fe) | |||||
25°C | Fe(OH)2 | 10-12.83 | -12.83 | 2 | 6.4 |
Fe(OH)3 | 10-2.59 | -2.59 | 3 | 0.9 | |
70°C | Fe(OH)2 | 10-10.77 | -10.77 | 2 | 5.4 |
Fe(OH)3 | 10-1.18 | -1.18 |
3 | 0.4 |