
 |
Atmospheric corrosion is an electrochemical process, requiring the presence of an electrolyte. Thin film "invisible" electrolytes tend to form on metallic surfaces under atmospheric corrosion conditions, when a certain critical humidity level is reached. For iron, this level is around 60%, in unpolluted atmospheres. The critical humidity level is not a constant - it depends on the corroding material, the hygroscopic nature of corrosion products and surface deposits and the presence of atmospheric pollutants.
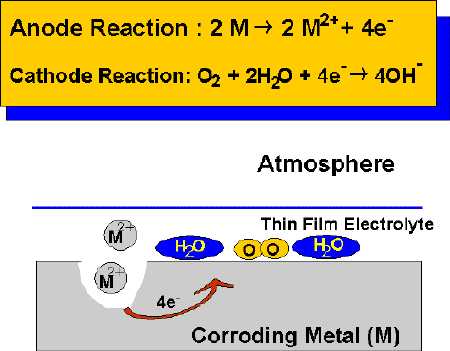
In the presence of thin film electrolytes, atmospheric corrosion proceeds by balancing anodic and cathodic reactions. The anodic oxidation reaction involves the dissolution of the metal in the electrolyte, while the cathodic reaction is often assumed to be the oxygen reduction reaction. Oxygen from the atmosphere is readily supplied to the electrolyte, under thin film corrosion conditions. The thickness and electrical conductivity of the film will greatly depend on relative humidity, the nature of the surface contaminants, and many other factors such as temperature, sunlight exposure etc. Some surface contaminants can be very hygroscopic, which means they will reduce the level of humidity causing the formation of a film of electrolyte and greatly increase the duration of wetness on a corroding surface.