
 |
Various methods have been developed for measuring many of the factors that influence atmospheric corrosion. The quantity and composition of pollutants in the atmosphere, the amount collected on surfaces under a variety of conditions, and the variation of these with time have been determined. Temperature, RH, wind direction and velocity, solar radiation, and amount of rainfall are easily recorded. Not so easily determined are dwelling time of wetness (TOW), and the quantity of sulfur dioxide and chloride contamination. However, methods for these determinations have been developed and are in use at various test stations. By monitoring these factors and relating them to corrosion rates, a better understanding of atmospheric corrosion can be obtained.
TOW is an estimated parameter based on the length of time when the relative humidity is greater than 80% at a temperature greater than 0oC. It can be expressed as the hours or days per year or the annual percentage of time.
A method of measuring the TOW has been developed by Sereda and correlated with the corrosion rates encountered in the atmosphere [9]. The moisture sensing elements in this sensor are manufactured by plating and selective etching of thin films of appropriate anode (copper) and cathode (gold) materials in an interlaced pattern on a thin nonconductive substrate as shown in the following Figure. When moisture condenses on the sensor it activates the cell, producing a small voltage (0 to 100 mV) across a 107 W resistor.
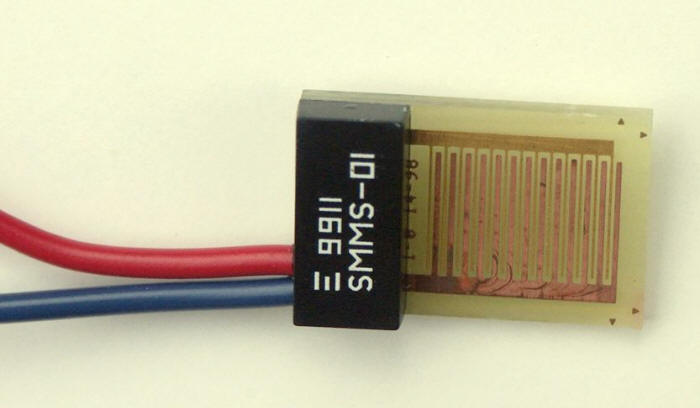
Interlocking combs of gold and copper electrodes in a ‘Sereda’ humidity sensor.
Thin sensing elements are preferred in order to preclude influencing the surface temperature to any extent. Although a sensor constructed using a 1.5-mm thick glass reinforced polyester base has been found to be satisfactory on plastic surfaces, this will not be the case with the same sensing element on a metal surface with a high-thermal conductivity. For metal surfaces, the sensing element should be appreciably thinner. Commercial epoxy sensor backing products of thickness of 1.5 mm, or less, are suitable for this purpose.
Sulfur dioxide is usually measured in terms of its concentration in air in units of µg m-3. Precise methods are available to monitor continuously the amount of sulfur dioxide in a given volume of air. However, this is only indirectly related to the effect of sulfur dioxide on corrosion since only the actual amount of hydrated sulfur dioxide or sulfur trioxide deposited on metal surfaces is important.
Since it is the SO2 deposited on the metal surface which affects the corrosion, it is also often measured in terms of deposition rate on the surface in units of mg/m2/day. The pollution levels can also be measured in terms of the concentration of the dissolved sulfate in rain water. The collection of sulfur dioxide by the lead peroxide cylinder method has been employed and seems to correlate with corrosion rates when combined with the TOW.
Airborne salinity refers to the content of gaseous and suspended salt in the atmosphere. It is measured by the concentration in the air in units of µg/m3. Since it is the salt that is deposited on the metal surface that affects the corrosion, it is often measured in terms of deposition rate in units of mg/m2/day. The chloride levels can also be measured in terms of the concentration of the dissolved salt in rain water.
A number of methods have been employed for determining the contamination of the atmosphere by aerosol transported chlorides, e.g. sea salt and road deicing salts. The ‘wet candle method,’ for example, is relatively simple, but has the disadvantage that it also collects particles of dry salt that might not be deposited otherwise. This technique uses a wet wick of a known diameter and surface area to measure aerosol deposition as shown in the following Figure.
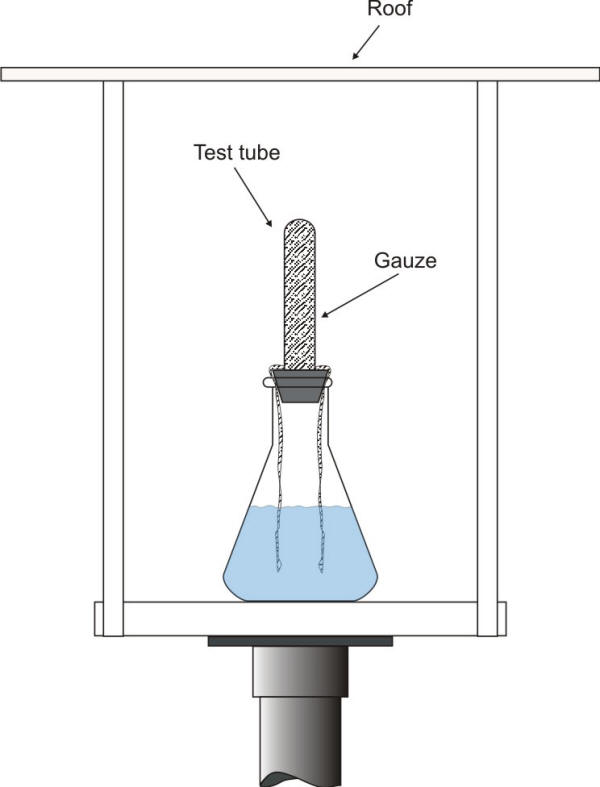
Schematic of a wet candle chloride apparatus.
The wick is maintained wet using a reservoir of water or 40% glycol/water solution. Particles of salt or spray are trapped by the wet wick and retained. At intervals, a quantitative determination of the chloride collected by the wick is made and a new wick is exposed.
In reality, the wet candle method gives an indication of the salinity of the atmosphere rather than the contamination of exposed metal surfaces. The technique is considered to measure the total amount of chloride arriving to a vertical surface and its results may not be truly significant for corrosivity estimates.
The simplest form of direct atmospheric corrosion measurement is by coupon exposures. Subsequent to their exposure, the coupons can be subjected to weight loss measurements, pit density and depth measurements and to other types of examination. Flat panels exposed on exposure racks are a common coupon-type device for atmospheric corrosivity measurements. Various other specimen configurations have been used, including stressed U-bend or C-ring specimens for stress corrosion cracking (SCC) studies. The main draw back associated with conventional coupon measurements is that extremely long exposure times are usually required to obtain meaningful data, even on a relative scale. It is not uncommon for such programs to run for 20 years or longer.
Some variations of the basic coupon specimens can provide rapid material/corrosivity evaluations. The helical coil adopted in the ISO 9226 methodology is a high surface area/weight ratio coupon that gives a higher sensitivity than panel coupons of the same material. The use of bimetallic specimens in which a helical wire is wrapped around a coarsely threaded bolt can provide additional sensitivity and forms the basis of the CLIMAT coupon for Classify Industrial and Marine Atmospheres.
An ASTM standard describes the construction of CLIMAT coupons. According to this standard CLIMAT coupons can be made from 1100 aluminum (UNS A91100) wire wrapped around threaded rods of nylon, 1010 mild steel (UNS G10100 or G10080), and CA110 copper (UNS C11000) [13]. The mass loss of aluminum wire after ninety days of exposure is considered to be an indication of atmospheric corrosivity. However, the relative corrosivity of atmospheres could be quite different for the various combinations of materials.
The aluminum wire on copper bolts has been found by many to be the most sensitive of the three proposed arrangements in the ASTM standard. While this arrangement is the most sensitive, the use of triplicate coupons on a single holder additionally provides an indication of the reproducibility of the measurements. A CLIMAT coupon with three copper rods was installed at the NASA Kennedy Space Center (KSC) beach corrosion test site shown the following Figure.

Aerial view of the NASA Kennedy Space Center beach corrosion test site where atmospheric corrosivity is the highest corrosivity of any test site in the continental United States.
The following Figures show the coupon shown immediately after it had been installed (a), after thirty days (b), and after sixty days (c).

a)

b)

c)
A CLIMAT coupon with three copper rods immediately after it was installed at the Kennedy Space Center beach corrosion test site a), after thirty days b), and after sixty days c).
KSC has the highest corrosivity of any test site in the continental United States. The mass loss recorded after a shorter exposure than usual can be very high. In the present example it was already 16% of the original aluminum wire after sixty days. The CLIMAT coupons sensitivity to atmospheric corrosivity can be used to study fluctuations on a micro-environmental scale.
| (previous) | Page 4 of 8 | (next) |