
 |
The oxygen content of any solution ranks high on the list of factors influencing the corrosion of iron and numerous other metals. Elimination of oxygen by deaeration is a practical means of reducing corrosion, as in the case of steam boilers which are operated with deaerated feed water. Differential aeration cells can be caused by crevices, lap joints, dirt and debris, and moist insulation. Under these conditions, the oxygen starved areas are anodic, while the areas with free access to oxygen are cathodic. Common terms for this type of corrosion include crevice corrosion, oxygen screening, and poultice action.
Oxygen not only enables a corrosion reaction by maintaining a cathodic reaction, but it can promote one. This occurs where there is a difference in the concentration of dissolved oxygen between two points of the same metal surface. Since the direction of the reaction is towards equilibrium, the only way that equilibrium can be approached by corrosion will be to reduce the concentration of oxygen where it is highest. Such reduction can be done by consuming the oxygen as shown in the following equation.
![]()
The result is that where there is a difference in the concentration of dissolved oxygen at two points on a metal surface, the surfaces in contact with the solution containing the higher concentration of dissolved oxygen will become cathodic to surfaces in contact with a lower concentration of dissolved oxygen that will in turn suffer accelerated corrosion as anodes in an oxygen concentration cell. It is easy to demonstrate an oxygen concentration cell with an experimental setup using two containers as shown in the following Figure.
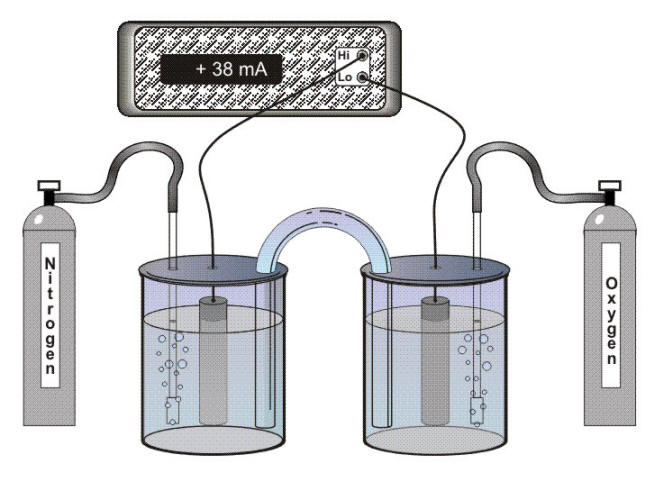
Experiment to demonstrate generation of a corrosion current by an oxygen concentration cell
In this experiment, pieces of steel electrically connected are immersed in a sodium chloride solution in the two containers. The solution in one container is saturated with oxygen and the solution in the other container is saturated with nitrogen. This establishes a difference in the concentration of dissolved oxygen in contact with the two pieces of steel. The high concentration of dissolved oxygen in one container makes the steel surface strongly cathodic to the steel in the other container. Dissolved oxygen concentration differences can also be established by velocity gradients and by crevices, but the location of anodes and cathodes from these sources is the opposite of a metal ion concentration cells. In the presence of a velocity gradient, more oxygen is brought to the surface moving at the highest velocity so that this surface becomes cathodic to any surface moving at a lower velocity because of the difference in oxygen availability.
In the following example the concentration cell is caused by differences in the electrolyte and in oxygen content around a buried anchor rod. The following Figure illustrates how soil stratification can produce an oxygen concentration cell on a tower anchor shaft. In this illustration, the upper soil layer is a loose somewhat gravelly soil below which there is a dense clay type soil.
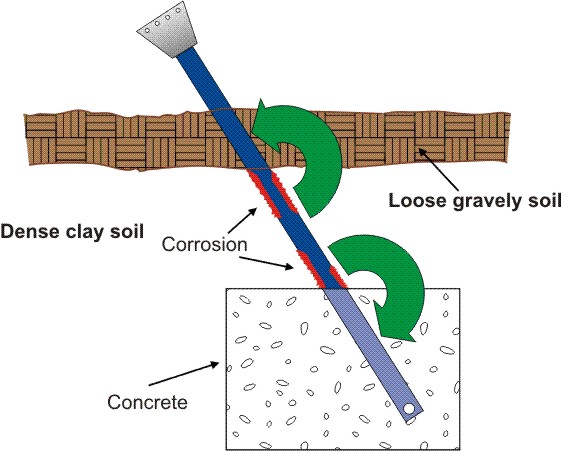
Differences in the porosity of the soil that can lead to an oxygen concentration corrosion cell. (Courtesy of Anchor Guard)
The portion of the shaft in contact with the clay type soil acts as an anode to the portion of the shaft in contact with the looser gravely soil, which is consequently the cathode. Again, we have a corrosion cell where the shaft deteriorates in the anodic areas. Such differential aeration cells are also very common on buried pipes. For example, a pipe usually rests on undisturbed soil at the bottom of a ditch. Around the sides and on top of the pipe is relatively loose backfill which has been replaced in the ditch.
Because the backfill is more permeable to oxygen (and the path is shorter) diffusing down from the surface, a cell is formed. In this case, the anode is the bottom surface of the pipe and the cathode is the rest of the surface. The electrolyte is the soil, and the connecting circuit is the metallic pipe itself. When a pipe or cable passes under paving, such as an airport runway, parking lot, or street such as illustrated in the following Figure, the portion under the paving has less access to oxygen than does the area lying under unpaved soil.
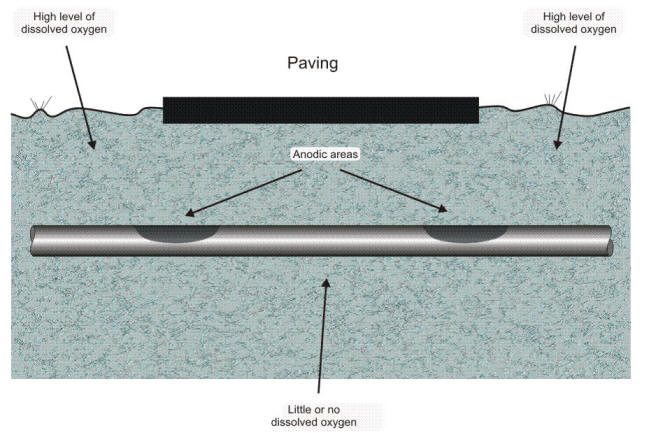
Oxygen differential cell resulting from burial under paving producing an oxygen concentration cell
In this particular example, although the entire length of pipe under the paving is anodic, most of the attack will take place close to the edge. Because the path through the electrolyte is shorter to this part, most of the current takes this low-resistance path. In this example the oxygen concentration cell components are:
The anode is the pipe under the paving
The cathode is the pipe outside the paving
The electrolyte is the soil
The connecting circuit is the pipe or cable
| (previous) | Page 7 of 14 | (next) |