
 |
The silver/silver chloride reference electrode is a widely used reference electrode because it is simple, inexpensive, very stable and non-toxic. As a laboratory electrode such as described in the following Figure, it is mainly used with saturated potassium chloride (KCl) electrolyte, but can be used with lower concentrations such as 1 M KCl and even directly in seawater.
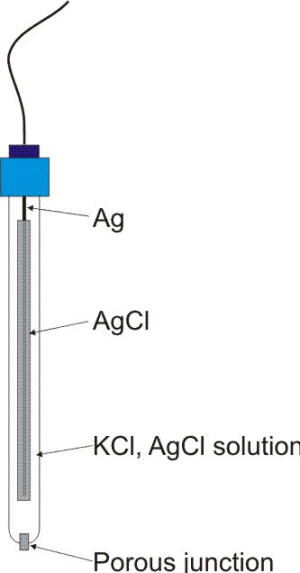
Schematic of a silver/silver chloride reference electrode
As indicated here, such changes in ionic concentrations also change the reference potential. Silver chloride is slightly soluble in strong potassium chloride solutions, so it is sometimes recommended that the potassium chloride be saturated with silver chloride to avoid stripping the silver chloride off the silver wire.(reference)
Typical laboratory electrodes use a silver wire that is coated with a thin layer of silver chloride either by electroplating or by dipping the wire in molten silver chloride. Industrial electrodes are fabricated using the same principle using other geometries such as planar electrodes. When the electrode is placed in a saturated potassium chloride solution it develops a potential of 199 mV vs. SHE. The potential of the half-cell reaction shown in equation is determined by the chloride concentration of the solution, as defined by the Nernst equation.
![]()
![]()
The silver-silver chloride reference electrode develops a potential proportional to the chloride concentration, whether it is sodium chloride, potassium chloride, ammonium chloride or some other chloride salt and remains constant as long as the chloride concentration remains constant.
| Most of reference electrodes use a saturated KCl solution with an excess of KCl crystals. The extra KCl dissolves into the electrolyte as the potassium and chloride ions diffuse out through the liquid junction in normal use. This extra buffer of KCl extends the time before the reference cell starts to drift due to the depletion of chloride ions in the electrolyte. The silver-silver chloride electrode simplicity of fabrication and fundamental ruggedness makes it a good candidate for many industrial applications where the electrochemical potential has to been measured or controlled. The following commercial electrode is specifically designed to be embedded in reinforced concrete. (Internet reference 74) | 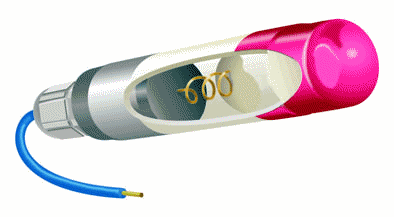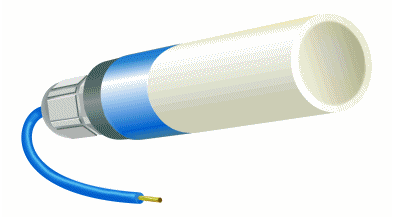 |
| (previous) | Page 7 of 17 | (next) |