
 |
In 1800, only months after the English chemist William Nicholson succeeded in decomposing water into hydrogen and oxygen by electrolysis, Johann Ritter duplicated the experiment but arranged the electrodes so that he could collect the two gases separately, thus improving on the experiments of Carlisle and Nicholson. Soon thereafter he discovered the process of electroplating. In 1800, Ritter observed that he could get metal to attach to copper which was the first electroplating attempt. He also observed the amount of metal deposited and the amount of oxygen produced during an electrolytic process that depended on the distance between the electrodes. He learned that the closer the electrodes, the stronger the effects. The basic concept of electrolysis and electroplating were discovered by Ritter at the same time or in some cases earlier than Carlisle and Nicholson's, Cruickanks', or Davy's experiments. In 1801 he observed thermoelectric currents and anticipated the discovery of thermoelectricity by Thomas Johann Seebeck.
William Hyde Wollaston was the first scientist to outline the differences between the new galvanic current and that of the standard frictional current when he presented a paper before the Royal Society in June 1801. He showed convincingly that the pile of Volta was electrical, had less tension (later called volts), but more quantity (later called current) than that of frictional electricity.
In 1802, William Cruickshank designed the first electric battery capable of mass production. Cruickshank had arranged square sheets of copper, which he soldered at their ends, together with sheets of zinc of equal size. These sheets were placed into a long rectangular wooden box that was sealed with cement. Grooves in the box held the metal plates in position. The box was then filled with an electrolyte of brine, or watered down acid. This flooded design had the advantage of not drying out with use and provided more energy than Volta's disc arrangement.
In 1802, Johann Ritter developed a dry cell battery from his efforts with electrolytic cells. He found that his new combination worked as well as the Volta pile to charge Leyden jars, and continued to function equally well for six days. Volta's pile worked only about 15 to 20 minutes before exhausting. Ritter again did not publish his work on the dry pile because he stated that his two months of very concentrated research would take him two years to write.
Giovanni Aldini, Italian physicist, was Galvani's nephew and the greatest supporter of Galvani's theory. Aldini's work contributed significantly to make Galvani's discoveries more widely known through a series of experiments that were described in details in Aldini's book published in London in 1803. This book provided an account of the late improvements in galvanism, with a series of curious and interesting experiments performed before the commissioners of the French National Institute, and repeated lately in the anatomical theaters of London. It was an influential book on galvanism, that presented for the first time a series of experiments in which the principles of Volta and Galvani were used together. The fine series of plates illustrated the experiments which involved bodies and heads of animals and humans. For the first time a description appears here of the magnetization of steel needles through connection to a voltaic circuit.
| The most famous of these experiments took place at the Royal College of Surgeons in London in 1803, on a hanged man named George Forster. Anatomical dissection had formed part of Forster’s death sentence, but no one could have visualized quite the violation that Aldini was going to inflict on him. Before a large medical and general audience, he took a pair of conducting rods linked to a powerful battery, and touched the rods to various parts of the body in turn. The results were dramatic. When the rods were applied to Forster’s mouth and ear, “the jaw began to quiver, the adjoining muscles were horribly contorted, and the left eye actually opened.” When one rod was moved to touch the rectum, the whole body convulsed: indeed, the movements were “so much increased as almost to give an appearance of re-animation”. | 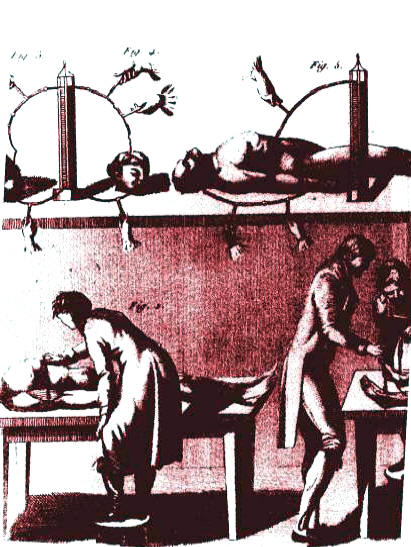 |
And so it went on, with Aldini moving the two rods around the body in a different combinations like a switchboard operator. According to newspaper reports of the time, some of the spectators genuinely believed that the body was about to come to life, and were suitably awestruck even though it did not happen. But Aldini himself gave no indication that he expected any such thing – although he did describe his ultimate aim as learning how to “command the vital powers.” In practice, he confined himself to concluding that galvanism “exerted a considerable power over the nervous and muscular systems.” He also noted that nothing could be done with the heart!
William Hyde Wollaston in association with Smithson Tennant carried experiments that lead to the production of platinum and platinum metals. Platinum had eluded the efforts of chemists to produce it. Tennant tried to produce platinum, but ended up discovering the new elements of iridium and osmium. Wollaston's effort, in turn, led him to the discovery of the new metals palladium (1803) and rhodium (1804). Wollaston named the metal Palladium (Pd) after Pallas (Athene), the second asteroid, discovered a year earlier. Pallas was the Greek goddess of wisdom. A second new metal, Rhodium, was obtained by neutralizing the aqua regia with caustic soda. He then found the process to produce malleable platinum in 1805 which earned him considerable money by 1826, and which apparently compensated him more than had his medical practice. The success of his method, which he kept secret until shortly before his death, yielded him financial independence for the rest of his life. He waited until 1828 to present a paper describing the process of platinum to the Royal Society.
| In 1809 Samuel Soemmering developed the first telegraph. He used a device with 26 wires (1 wire for each letter of the German alphabet) terminated in a container of acid. At the sending station, a key, which brought a battery into the circuit, was connected as required to each of the line wires. The passage of a current caused the acid to decompose chemically and the message was read by observing at which of the terminals the bubbles of gas appeared. This is how he was able to send messages, one letter at a time. | 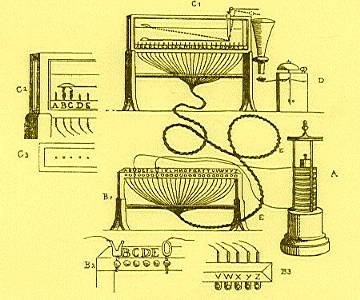 |
Sir Humphry Davy work with electrolysis led to conclude that the production of electricity in simple electrolytic cells resulted from chemical action and that chemical combination occurred between substances of opposite charge. He therefore reasoned that electrolysis, the interactions of electric currents with chemical compounds, offered the most likely means of decomposing all substances to their elements. These views were explained in 1806 in his lecture "On Some Chemical Agencies of Electricity," for which, despite the fact that England and France were at war, he received the Napoleon Prize from the Institut de France (1807).
This work led directly to the isolation of sodium and potassium from their compounds (1807) and of the alkaline-earth metals from theirs (1808). He also discovered boron (by heating borax with potassium), hydrogen telluride, and hydrogen phosphide (phosphine). He showed the correct relation of chlorine to hydrochloric acid and the untenability of the earlier name (oxymuriatic acid) for chlorine; this negated Lavoisier's theory that all acids contained oxygen. He explained the bleaching action of chlorine (through its liberation of oxygen from water) and discovered two of its oxides (1811 and 1815), but his views on the nature of chlorine were disputed. He was not aware that chlorine is a chemical element, and experiments designed to reveal oxygen in chlorine failed.
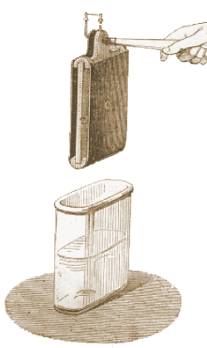 | William Hyde Wollaston made improvements to the galvanic pile in 1813 or1815. In Wollaston's battery the copper plates were doubled (a copper plate bent round into a U-shape) with a single plate of zinc placed in the center of the bent copper. The zinc plate was prevented from making contact with the copper by pieces or dowels of cork or wood. In his single cell design, the copper U-shaped plate was welted to a horizontal handle for lifting the copper and zinc plates from the activating solution when the battery was not in use. The metal plates and the solution were contained in an earthenware vessel. His design was the best battery at the time. |
Hans Christian Ørsted discovery of the magnetic effect of electrical currents in 1820 was immediately recognized as an epoch-making advance, although he left further work on electromagnetism to others. André-Marie Ampère quickly repeated Oersted's experiment, and formulated them mathematically. Ørsted also discovered that not only is a magnetic needle deflected by the electric current, but that the live electric wire is also deflected in a magnetic field, thus laying the foundation for the construction of the electric motor. In addition to electromagnetism Ørsted worked on the compressibility of gases and liquids and on diamagnetism. Ørsted's discovery (1820) of piperine, one of the pungent components of pepper, was an important contribution to chemistry, as was his preparation of metallic aluminum in 1825.
In 1820, elaborating on the work of H.C. Ørsted, Dominique Arago showed that the passage of an electric current through a cylindrical spiral of copper wire caused it to attract iron filings as if it were a magnet and that the filings fell off when the current ceased. Arago had just invented the electromagnet. During the same experiements Arago observed that iron was magnetized only during the flow of current, the effect finished when the current stopped. This discovery became thebasis of Morse telegraph.
Robert Hare developed in 1820 the Deflagrator, a form of voltaic battery having large plates used for producing rapid and powerful combustion. A modified form of this apparatus was employed in 1823 in volatilizing and fusing carbon. It was with these batteries that the first application of voltaic electricity to blasting under water was made in 1831.
In 1820, Johann Schweigger built a rectangular wooden frame on which he wound an insulated wire. This was called the Schweigger multiplier. A magnetic needle was suspended from a thin thread inside the coil. In the absence of electrical current the needle is oriented according to the magnetic meridian. When an electrical current is passed through the coil on the frame, the needle changes direction and the stronger the current, the more marked the deflection.
In the early 1820's, André-Marie Ampère attempted to develop a combined theory of electricity and magnetism after hearing about experimental results by the Danish physicist Hans Christian Ørsted. Ampère formulated a circuit force law and treated magnetism by postulating small closed circuits inside the magnetized substance. Ampère was also the first to develop measuring techniques for electricity with an instrument utilizing a free-moving needle to measure the flow of electricity. Its later refinement was known as the galvanometer. He used a highly sensitive galvanometer to make his measurements.
In 1821, Estonian-German physicist Thomas Johann Seebeck demonstrated the electrical potential in the juncture points of two dissimilar metals when there is a heat difference between the joints. He joined a copper wire with a bismuth wire to form a loop or circuit. Two junctions were formed by connecting the ends of the wires to each other. He then accidentally discovered that if he heated one junction to a high temperature, and the other junction remained at a cooler temperature a magnetic field was observed around the circuit of different temperatures.
He did not recognize, believe, or report that an electrical current was being generated when heat was applied to one junction of the two metals. He used the term thermomagnetic currents or thermomagnetism to express his discovery. During the following two years, 1822-1823, he reported on his continuing observations to the Prussian Academy of Sciences, where he describes this observation as "the magnetic polarization of metals and ores produced by a temperature difference." This effect became the basis of the thermocouple that still considered the most accurate measurement of temperature today.
| In 1822 Peter Barlow built a device which is to be considered one of the first models of an electric motor supplied by continuous current. Mercury is poured into the trough located on the base of the apparatus. The wheel is lowered until a spoke just dips into the mercury. Voltage applied to the binding posts will cause rotation of the wheel. |
|
What is now known as Ohm's law appeared in this famous book "Die galvanische Kette, mathematisch bearbeitet" (The Galvanic Circuit Investigated Mathematically) (1827) in which he gave his complete theory of electricity. The book begins with the mathematical background necessary for an understanding of the rest of the work. While his work greatly influenced the theory and applications of current electricity, it was coldly received. We should remark here that such a mathematical background was necessary for even the leading German physicists to understand the work, for the emphasis at this time was on a non-mathematical approach to physics. We should also remark that, despite Ohm's attempts in this introduction, he was not really successful in convincing the older German physicists that the mathematical approach was the right one.
In 1829 Antoine-César Becquerel developed the Constant Current Cell, which was the forerunner of the well-known "Daniell cell". When this acid-alkali cell was monitored by a galvanometer, current was found to be constant for an hour, the first instance of “constant current”. His differential galvanometer increased he accuracy to be attained in the measurement of electrical resistances. He applied the results of his study of thermoelectricity to the construction of an electric thermometer and measured with it the temperature of the interior of animals, of the soil at different depths, of the atmosphere at different heights. He was also very much interested in questions of meteorology, climate, and agriculture. Becquerel enjoyed success in many areas. He helped validate Faraday's laws and conduct extensive investigations on the electrodeposition of metals with applications for metal finishing and metallurgy. Solar cell technology dates to 1839 when Antoine-César Becquerelobserved that shining light on an electrode submerged in a conductive solution would create an electric current.
Michael Faraday began, in 1832, what promised to be a rather tedious attempt to prove that all electricities had precisely the same properties and caused precisely the same effects. The key effect was electrochemical decomposition. Voltaic and electromagnetic electricity posed no problems, but static electricity did. As Faraday delved deeper into the problem, he made two startling discoveries. First, electrical force did not, as had long been supposed, act at a distance upon chemical molecules to cause them to dissociate. It was the passage of electricity through a conducting liquid medium that caused the molecules to dissociate, even when the electricity merely discharged into the air and did not pass into a "pole" or "centre of action" in a voltaic cell. Second, the amount of the decomposition was found to be related in a simple manner to the amount of electricity that passed through the solution.
These findings led Faraday to a new theory of electrochemistry. The electric force, he argued, threw the molecules of a solution into a state of tension. When the force was strong enough to distort the fields of forces that held the molecules together so as to permit the interaction of these fields with neighboring particles, the tension was relieved by the migration of particles along the lines of tension, the different species of atoms migrating in opposite directions. The amount of electricity that passed, then, was clearly related to the chemical affinities of the substances in solution. These experiments led directly to Faraday's two laws of electrochemistry:
The amount of a substance deposited on each electrode of an electrolytic cell is directly proportional to the quantity of electricity passed through the cell
The quantities of different elements deposited by a given amount of electricity are in the ratio of their chemical equivalent weights