
 |
Acid rain is a general name for many phenomena including acid fog, acid sleet, and acid snow. The father of acid rain research is an Englishman named Charles Angus Smith who suggested in, 1852, that sulfuric acid in Manchester, English, was causing metal to rust and dyed goods to fade. One source that causes acid rain are fossil fuel. (reference)
Although we associate the acid threat with rainy days, acid deposition occurs all the time, even on sunny days. Acid Deposition is the scientific term used to describe "Acid Rain". When atmospheric pollutants such as sulfur dioxide and nitrogen oxides mix with water vapor in the air, they are converted to sulfuric and nitric acids. These acids make the rain acidic, hence the term "acid rain". Rain returns the sulfur and nitrogen acids to Earth, and in high concentrations, can cause damage to natural environments including forests and freshwater lakes. This form of acid deposition is known as wet deposition.
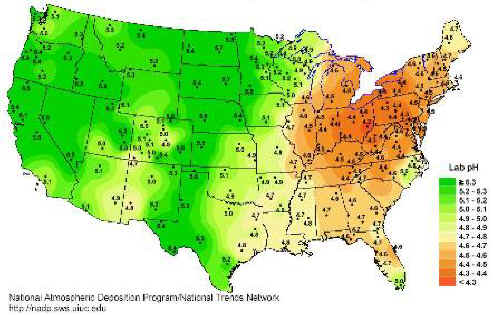
Average measured acidity in precipitations (USA) recorded for 1999 (reference)