
 |
The SHE is the universal reference for reporting relative half-cell potentials. It is a type of gas electrode and was widely used in early studies as a reference electrode, and as an indicator electrode for the determination of pH values. The SHE could be used as either an anode or cathode depending upon the nature of the half-cell it is used with. The SHE consists of a platinum electrode immersed in a solution with a hydrogen ion concentration of 1.00M. The platinum electrode is made of a small square of platinum foil which is platinized (known as platinum black). Hydrogen gas, at a pressure of 1 atmosphere, is bubbled around the platinum electrode. The platinum black serves as a large surface area for the reaction to take place, and the stream of hydrogen keeps the solution saturated at the electrode site with respect to the gas. It is interesting to note that even though the SHE is the universal reference standard, it exists only as a theoretical electrode which scientists use as the definition of an arbitrary reference electrode with a half-cell potential of 0.00 volts. (Because half-cell potentials cannot be measured, this is the perfect electrode to allow scientists to perform theoretical research calculations.) The reason this electrode cannot be manufactured is due to the fact that no solution can be prepared that yields a hydrogen ion activity of 1.00M.
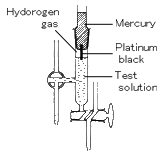
hydrogen electrode is made by adding platinum black to platinum wire or a platinum plate. It is immersed in the test solution and an electric charge is applied to the solution and platinum black with hydrogen gas. The hydrogen-electrode method is a standard among the various methods for measuring pH. The values derived using other methods become trustworthy only when they match those measured using hydrogen electrode method. However, this method is not appropriate for daily use because of the effort and expense involved, with the inconvenience of handling hydrogen gas and great influence of highly oxidizing or reducing substances in the test solution. (reference)