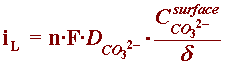|
The value of calcareous deposits in the effective and efficient operation of marine cathodic protection systems is generally recognized by corrosion engineers. The calcareous films are known to form on cathodic metal surfaces in seawater, thereby enhancing oxygen concentration polarization and reducing the current density needed to maintain a prescribed cathodic potential. For most cathodic surfaces in aerated waters, the principal reduction reaction is described by the following reaction:
O2 + 2 H2O + 4 e- --> 4 OH-
In cases where the potential is more negative than the reversible hydrogen electrode potential, the production of hydrogen as described in the following reaction becomes possible:
2 H2O + 2 e- --> H2 + 2 OH-
In either case, the production of hydroxyl ions results in an increase in pH for the electrolyte adjacent to the metal surface. In other terms, an increase in OH- is equivalent to a corresponding reduction in acidity or H+ ion concentration. This situation causes the production of a pH profile in the diffuse layer where the equilibrium reactions can be quite different from the bulk seawater conditions.
Temperature, relative electrolyte velocity and electrolyte composition will all influence this pH profile. There is both analytical and experimental evidence that such a pH increase exists as a consequence of the application of a cathodic current. In seawater, pH is controlled by the carbon dioxide system described in the following interrelated reactions:
CO2 + H2O --> H2CO3
H2CO3 --> H+ + HCO3-
HCO3- --> H+ + CO32-
If OH- is added to the system as a consequence of one of the above cathodic processes the pH adjacent to a metallic surface will increase favoring the precipitation of a calcareous deposit according to the following reactions:
CO2 + OH- --> HCO3-
OH- + HCO3- --> H2O + CO32-
CO32- + Ca2+ --> CaCO3(s)
The equilibria represented by Equations (3) through (8) further indicate that as OH- is introduced (Equation (1) and/or (2)) and reacts (Equations (6) and (7)), then Equations (4) and (5) are displaced to the right, resulting in proton (H+) production. This opposes any rise in pH and accounts for the buffering capacity of seawater. Irrespective of this, however, Equations (3) through (8) indicate that this buffering action is accompanied by the formation of calcareous deposits on cathodic surfaces exposed to seawater.
Magnesium compounds, Mg(OH)2 in particular, could also contribute to the protective character of calcareous deposits. However, calcium carbonate is thermodynamically stable in surface seawater, where it is supersaturated, while magnesium hydroxide is unsaturated and less stable. In fact, Mg(OH)2 would precipitate only if the pH of seawater was to exceed approximately 9.5. It is the main reason why the behavior of CaCO3 in seawater has been so extensively studied since calcium carbonate sediments are prevalent and widespread in the oceans.
It has been demonstrated that that calcium carbonate occurs in the oceans in two crystalline forms, i.e. calcite and aragonite. Partly because calcite and magnesium carbonate have similar structures, these compounds form solid solutions, the Ca:Mg ratio of which depends on the ratio of these ions in seawater. Theoretical calculations suggest that calcite in equilibrium with seawater should contain between 2 and 7 mol% MgCO3. But although low magnesium calcite is the most stable carbonate phase in seawater, its precipitation and crystal growth are strongly inhibited by dissolved magnesium. Consequently aragonite is the phase that actually precipitates when seawater is made basic by the addition of sodium carbonate.
The degree of saturation for aragonite is described by the following solubility or equilibrium constant:
Ksp, aragonite = (Ca2+)×(CO32-)
where (Ca2+) and (CO32-) are the molalities of the Ca2+ and CO32- ions respectively.
At 25oC Ksp, aragonite = 6.7 x 10-7
In order to understand the build-up of carbonate ions at a metallic surface under cathodic protection (CP), one can combine Equations (2), (6) and (7) to obtain an expression describing the electrochemical production of carbonate ions (Equation (10)).
H2O + CO2 + 2e- --> H2 + CO32-
By referring to the section of the Kinetics Module on concentration overpotential, one can the develop an expression for the limiting current corresponding to this reaction:
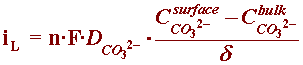
where, at neutral bulk pH, the concentration of carbonate ions in seawater is basically zero, and the expression of iL can be correctly described by: