
 |
The efficiency of a galvanic anode is the ratio of an anode weight sacrificed for CP purposes divided by the total theoretical ampere-hours or capacity of the material used. Galvanic anode materials are subject to self-corrosion which uses some of its energy. This is why the efficiency is less than 100%.
A prospective sacrificial anode must possess a large number of electrons per unit mass and should deliver these electric charges efficiently. Thus the electrical output of an anode is given by current capacity which is expressed in Ah kg-1 or kg A-1 y-1. The value of the current capacity is determined by the electrochemical equivalent, the density and the efficiency of the anodic material. The electrochemical equivalent, which is dependent on the atomic weight and valence, is a characteristic of the anode material.
For example, pure zinc has a theoretical maximum capacity of 820 Ah per kilogram (373 Ah per pound). This means that if a zinc anode were to discharge one ampere continuously, one kilogram would be consumed in 820 hours. If this kilogram was discharging one tenth of an ampere, it would be totally consumed in 8200 hours or 48 weeks. Actually, zinc anodes operate, typically, at about 95 % efficiency. This means that the energy content available for useful current output would be 820 x 0.95, or 779 Ah per kg.
Another way of expressing this is in terms of kg per ampere-year. At 779 Ah per kg useful output for zinc, the conversion would be:
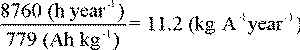
The actual anode efficiency is determined by a number of factors including nature of the environment, operating current density and metallurgical microstructure. It is apparent that if the cathode reaction rate on the anode is low then the efficiency will be high, so that there is minimum self corrosion. Similarly large operating currents will yield high anode efficiency. It should be added that the type of corrosion attack experienced by the anode also significantly affects the magnitude of the anode efficiency. For instance, severe pitting and intergranular attack may result in a chunk of the anode to become detached without complete consumption of the electric charge in that piece.