
 |
The conductivity of an environment can itself be a complex function. When a salt dissociates, the resulting ions interact with surrounding water molecules to form charged clusters known as solvated ions. These ions can move through the solution under the influence of an externally applied electric field. Such motion of charge is known as ionic conduction and the resulting conductance is the reciprocal function of the resistance of an environment.
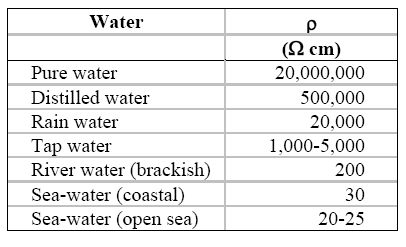
The dependence upon the size and shape of the conductor can be corrected by using the resistivity r rather than the resistance R, as expressed in equation for the simple cell shown in the following Figure and the Table under it lists some typical values of water resistivity.
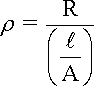
where:
R is the measured resistance across the cell
A is the cross-sectional area of each electrode, provided that both electrodes have the same dimensions
l is the gap separating the electrodes in the following Figure
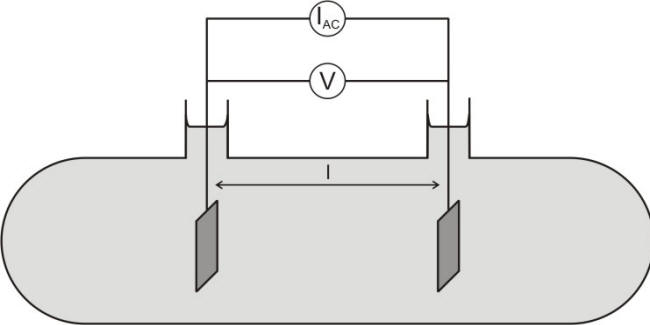
Schematic of conductivity cell containing an electrolyte and two inert electrodes of surface A parallel to each other and separated by distance l
Resistivity of some typical waters
The ratio (l/A) is also called the cell constant or ‘shape factor’ and has units of cm-1 or m-1. The electrochemical cell shown in the previous Figure is commonly used to evaluate the conductivity of a solution between two electrodes by using an alternating current technique. Other geometries would require the calculation of appropriate cell constants.
Explain the main differences between the ohmic drop in an aqueous environment and the ohmic drop in an electrical conductor.
The cell constant of an electrochemical cell with two concentric tubes as electrodes would be, for example, expressed by equation. Such an arrangement is a common design in the production of domestic water heaters in which a central sacrificial magnesium anode is inserted to protect the surrounding tank material.
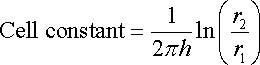
where:
h is the height of the cylinders
r2 and r1 are respectively the internal and external cylinder radii
The ohmic drop can be minimized, when carrying out electrochemical tests, by putting the reference electrode in a Luggin capillary brought as close as possible to the surface being monitored in order to minimizes any ohmic drop in the electrolyte associated with the passage of current in the cell.
Additionally, the Luggin capillary allows sensing of the solution potential close to the working electrode without the adverse shielding effects that may be caused when the reference electrode is positioned in front of the surface being monitored. A Luggin capillary can be made of any material provided it is inert to the electrolytic environment. It basically consists of a bent tube generally filled with the test solution with a large enough opening to accommodate a reference electrode and a usually much smaller opening at the other end to provide diffusion movement of the electrolyte.
| (previous) | Page 6 of 10 | (next) |