
 |
Antimony is a unique metal with the characteristic of a direct relationship between pH and its measured potential. The potential difference or voltage developed between antimony and a copper/copper sulfate reference electrode varies between approximately 0.1 volts to 0.7 volts due to variations in the pH. (reference)
The antimony electrode must be cleaned prior to use. As with any other half-cells, special cleaning procedures must be used. Antimony is very brittle and must be treated carefully. The antimony tip must be kept smooth, and there must be no rough surface or pits.
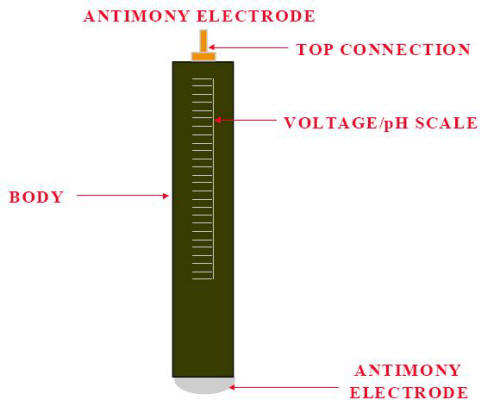
Antimony electrode
The antimony pH electrode is particularly suited for solutions containing hydrofluoric acid (HF) since the sensor has no glass wetted parts and will not degrade in such environment.
| (previous) | Page 12 of 17 | (next) |