
 |
The standard hydrogen half-cell is rather awkward to use under many circumstances in which potential measurements are to be made. The other half-cells most frequently used in corrosion studies, along with their potentials relative to the standard hydrogen half-cell, are listed in the following Table. (reference)
Equilibrium potential of the main reference electrodes used in corrosion, at 25oC
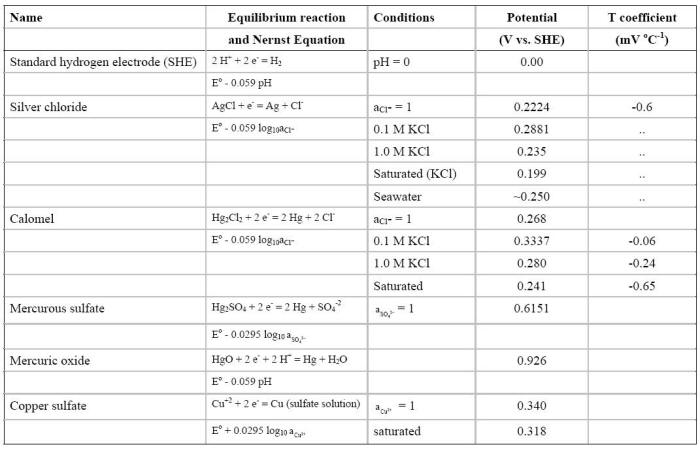
Most reference electrodes are used with a saturated solution and an excess of the salt crystals. The extra salt dissolves into the half-cell solution as some of the ions diffuse out of the reference cell body through the liquid junction during normal use. This extra buffer of salt extends the time before the reference cell starts to drift due to the depletion of ions as predicted by Nernst equation.
| (previous) | Page 5 of 17 | (next) |