
 |
With this widely used technique in corrosion monitoring, the polarization resistance of a material is defined as the slope of the potential-current density (DE/Di) curve at the free corrosion potential, yielding the polarization resistance Rp that can be related (for reactions under activation control) to the corrosion current by the Stern-Geary equation: (reference)
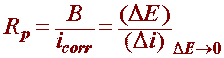
where:
Rp is the polarization resistance
icorr the corrosion current
The proportionality constant , B, for a particular system can he determined empirically (calibrated from separate weight loss measurements) or, as shown by Stern and Geary, can be calculated from ba and bc, the slopes of the anodic and cathodic Tafel
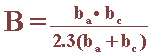
The Tafel slopes themselves can be evaluated experimentally using real polarization plots. The corrosion currents estimated using these techniques can be converted into penetration rates using Faraday's law or a generic conversion chart.
The study of uniform corrosion or studies assuming corrosion uniformity are probably the most widespread application of electrochemical measurements both in the laboratory and in the field. The widespread use of these electrochemical techniques does not mean that they are without complications. Both linear polarization and Tafel extrapolation need special precautions for their results to be valid. The main complications or obstacles in performing polarization measurements can be summarized in the following categories:
Effect of Scan Rate: The rate at which the potential is scanned may have a significant effect on the amount of current produced at all values of potential. The rate at which the potential is changed, the scan rate, is an experimental parameter over which the user has control. If not chosen properly, the scan rate can alter the scan and cause a misinterpretation of the features.
Effect of Solution Resistance: The distance between the Luggin capillary (of the salt bridge to the reference electrode) and the working electrode is purposely minimized in most measurements to limit the effect of the solution resistance. In solutions that have extremely high resistivity, this can be an extremely significant effect.
Changing Surface Conditions: Since corrosion reactions take place at the surface of materials, when the surface is changed, due to processing conditions, active corrosion or other reasons, the potential is usually also changed. This can have a strong effect on the polarization curves.
Determination of Pitting Potential: In analyzing polarization curves the appearance of a hysteresis (or loop) between the forward and reverse scans is often thought to denote the presence of localized corrosion (pitting or crevice corrosion).