
 |
This Module consists of fourteen Web pages of required reading. The pagination is visible at the bottom of each page with direct links to adjacent pages.
Additional information can be found in sections 7.1 to 7.6 of the reference textbook (Corrosion Engineering: Principles and Practice).
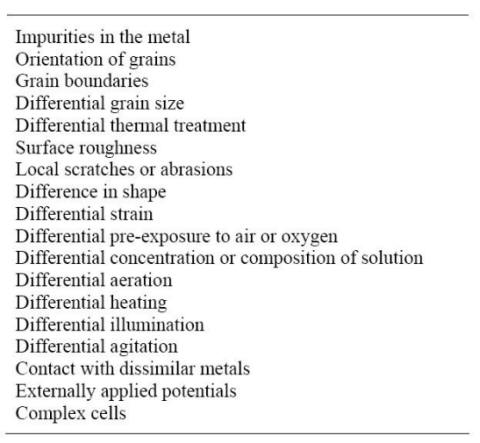
As described in the previous Chapter, corrosion damage may take various forms which are themselves triggered by apparently different compounding factors. The initiation and progression of corrosion processes indeed depend on the complex interaction of a multitude of factors such as:
The nature of the driving forces behind these factors has been the subject of scientific studies by many scientists in the early part of the twentieth century. In a landmark paper, Mears and Brown have summarized 18 mechanisms, listed in the following Table, by which differences in potential may develop on metal surfaces [1].
Causes of corrosion currents
There is a vast body of information relating practically all the previously listed factors to actual field observations and subsequent analysis of failed components. These failure investigations are typically carried out in a detailed mechanistic ‘bottom-up’ manner whereby a failed component would be sent to the laboratory where analytical techniques would then be used following well established protocols. Chemical analysis, hardness testing, metallography, optical and electron microscopy, fractography, X-ray diffraction and surface analysis are specialized tools used in such investigations.
However, this approach alone provides little or no insight into the real causes of failure. Underlying causes of serious corrosion damage that can often be cited include human factors such as lack of corrosion awareness, inadequate training and poor communication. Further underlying causes may include weak maintenance management systems, insufficient repairs due to short term profit motives, a poor organizational ‘safety culture’, defective supplier's products, or an incorrect material selection.
It is thus apparent that there can be multiple causes associated with a single corrosion mechanism. Clearly, a comprehensive failure investigation providing information on the root cause of failure is much more valuable than one merely establishing the corrosion mechanism(s).Establishing the real causes of corrosion failures (often related to human behavior) is a much harder task than merely identifying the failure mechanisms.
In contrast to the traditional scientific mechanistic approach, systems engineers prefer the ‘top-down’ approach that broadens the definition of the system and is more likely to include causes of corrosion failures such as human behavior.This is consistent with the lessons to be learned from the UK Hoar Report which stated that corrosion control of even small components could result in major cost savings because of the effect on systems rather than just the components [2].
| (previous) | Page 1 of 14 | (next) |
See also CCE 513: Corrosion Engineering
Mears RB, Brown RH. Designing to Prevent Corrosion. Corrosion 1947; 3: 97-120.
Hoar, T. P. Report of the Committee on Corrosion and Protection.1971. London, UK, Her Majesty's Stationary Office.
Standard Guide for Applying Statistics to Analysis of Corrosion Data. In: Annual Book of ASTM Standards. Philadelphia, PA: American Society for Testing of Materials, 1999.
Tomiura A. Lessons for a Case Study of Property Databases in Materials Development. In: Nishijima S, Iwata S, Newton CH, eds. Computerization/Networking of Materials Databases, STP 1311. Philadelphia, PA: American Society for Testing and Materials, 1996; 3-20.
Dexter SC. Microbiologically Influenced Corrosion. In: Cramer DS, Covino BS, eds. Volume 13A: Corrosion: Fundamentals, Testing, and Protection. Metals Park, OH: ASM International, 2003; 398-416.
Koch, G. H, Brongers, M. P. H., Thompson, N. G., Virmani, Y. P., and Payer, J. H. Corrosion Costs and Preventive Strategies in the United States. FHWA-RD-01-156. 2001. Springfield, VA, National Technical Information Service.
Cowan RL, Staehle RW. The Thermodynamics and Electrode Kinetic Behavior of Nickel in Acid Solution in the Temperature Range 25o to 300oC. Journal of the Electrochemical Society 1971; 118: 557-68.
Staehle RW. Lifetime Prediction of Materials in Environments. In: Revie RW, ed. Uhlig's Corrosion Handbook. NY, NY: Wiley-Interscience, 2000; 27-84.
Staehle RW. Environmental Definition. In: Revie RW, Sastri VS, Ghali E, Piron DL, Roberge PR, eds. New York, NY: Pergamon Press, 1991; 3-43.
Bell, G. E. C., Schiff, M. J., and Wilson, D. F. Field Observations and Laboratory Investigations of Thermogalvanic Corrosion of Copper Tubing. CORROSION 97, Paper # 568. 1997. Houston, TX, NACE International.
Al-Darbi, M. M., Agha, K., and Islam, M. R. Modeling and Simulation of the Pitting Microbiologically Influenced Corrosion in Different Industrial Systems. CORROSION 2005, Paper # 505. 2005. Houston, TX, NACE International.
Hill EC. Microbial Aspects of Metallurgy. New York, NY: American Elsevier, 1970. 13. Kobrin G. Catalog of Color Photographs of MIC. In: Kobrin G, ed. Microbiologically Influenced Corrosion. Houston, TX: NACE International, 1993; 90-100.
Kobrin G. Catalog of Color Photographs of MIC. In: Kobrin G, ed. Microbiologically Influenced Corrosion. Houston, TX: NACE International, 1993; 90-100.