
 |
Concentration polarization is the polarization component that is caused by concentration changes in the environment adjacent to the surface as illustrated in the following Figure. When a chemical species participating in a corrosion process is in short supply, the mass transport of that species to the corroding surface can become rate controlling. (reference)
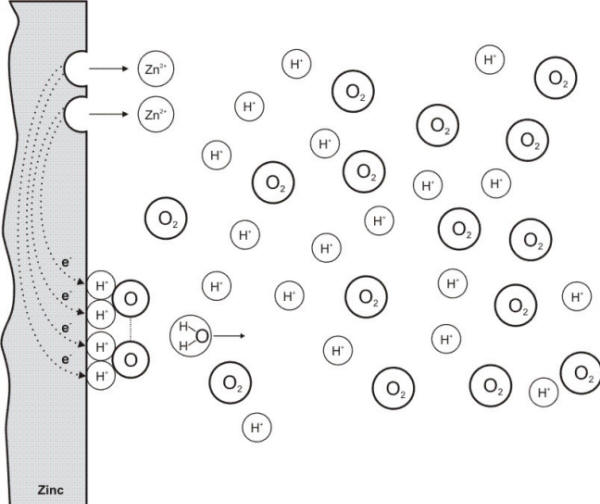
Concentration changes in the vicinity of an electrode causing a concentration polarization effect
Describe a simple method to verify if an electrochemical reaction is limited by a concentration polarization effect.
A frequent case of concentration
polarization occurs when the cathodic processes depend on the reduction of dissolved
oxygen since it is usually in low concentration, i.e.
in parts per million (ppm).
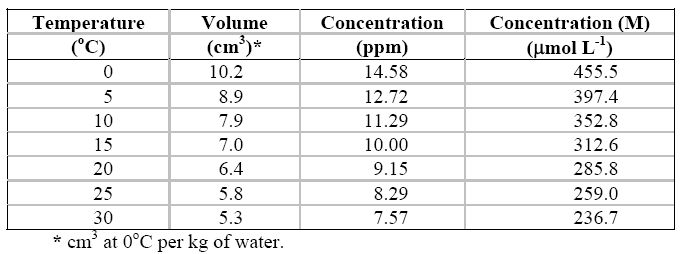
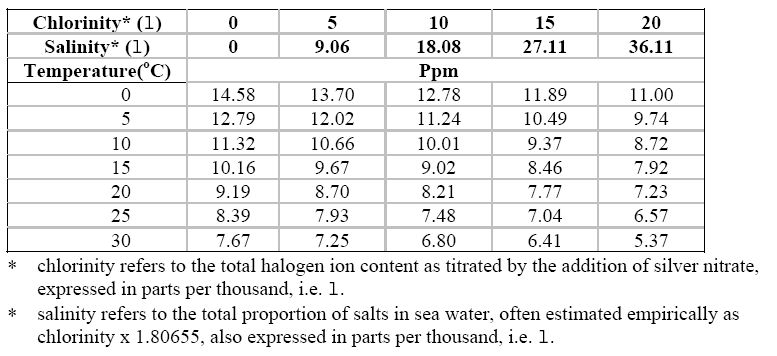
The following Tables
contain respectively
data related to the solubility of oxygen in
air saturated water at different temperatures and
data on the solubility of oxygen in seawater
of different salinity, chlorinity, and temperatures. In both Tables, the level of
dissolved oxygen is seen to increase as the temperature decreases.
Solubility of oxygen in air saturated water
Oxygen dissolved in seawater in equilibrium with a normal atmosphere
How many grams of dissolved oxygen are present in one liter of aerated water at 5ºC? ... at at 30ºC?
Describe a simple method to reduce the quantity of dissolved oxygen in a water container or vessel.
As illustrated in the following
Figure, mass transport to a surface is
governed by three forces, i.e diffusion,
migration and
convection.
In the absence of an electrical field, the migration term, that only affects
charged ionic species, is negligible while the convection force disappears in stagnant
conditions.
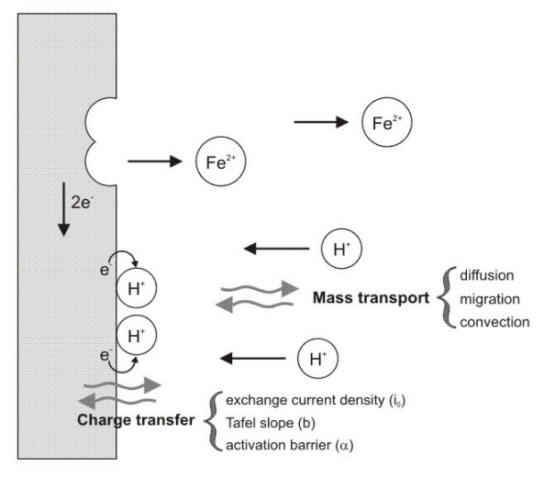
Graphical representation of the processes occurring at an electrochemical interface
For purely diffusion controlled
mass transport, the flux of a species O to a surface from the bulk is described
with Fick’s
first law:
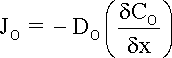
where:
JO
is the flux of species O (mol s-1 cm-2)
DO
is the diffusion coefficient of species O (cm2 s-1)
dCO
is the concentration gradient of species O across the interface between the metallic
surface and the bulk environment (mol cm-3)
dx is
the thickness of the interface (cm).
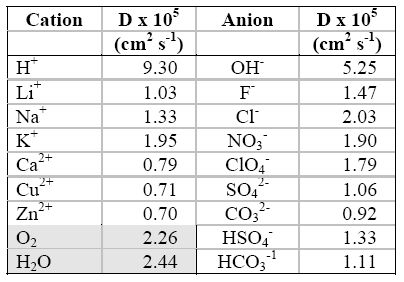
The following Table contains values
for DO of some common ions.
For more practical situations the diffusion coefficient can be approximated
with the help of equation, that relates DO
to the viscosity of the solution (m)
and absolute temperature:
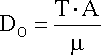
where
A is a constant for the system.
Diffusion coefficients of selected ions at infinite dilution in water at 25oC
The following Figure illustrates the concentration-distance profile at the electrode surface approximated by a simple gradient. In this diagram the metallic surface is positioned at the ordinate axis while the x-axis expresses the distance away from the electrode and the y-axis the concentration of the chemical species being reacted.
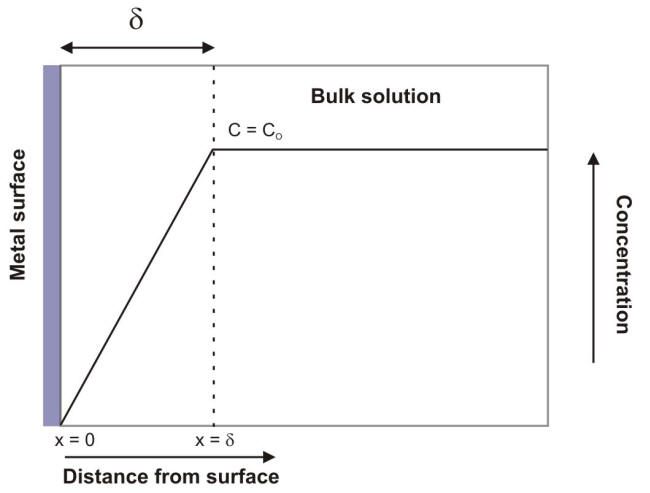
Nernst diffusion layer for a limiting current situation
For well mixed solutions, the concentration is constant in the bulk or convective region. This is represented by the horizontal line where C = CO. There is also a region where the concentration drops, falling to zero at the electrode surface. The Nernst diffusion layer, also called the diffuse layer, associated with this drop has a specific thickness (d) that depends upon the nature of the solution into which it extends. For stirred aqueous solutions the thickness of the diffuse layer varies between 0.01 and 0.001 mm.
For a chemical species
O that is consumed by the cathodic reaction at the corroding surface, the concentration
gradient (dCO/dx)
is greatest when the concentration of that species is completely depleted at the
surface, i.e. CO
= 0. It follows that the cathodic current is limited in that condition,
as expressed by equation:
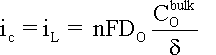
For intermediate
cases, i.e. when the cathodic current is smaller than
iL,
hconc
can be evaluated using an expression derived from Nernst equation:
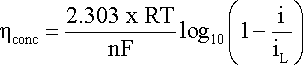
where 2.303·R·T/F
= 0.059 V when T = 298.16 K.
| (previous) | Page 4 of 10 | (next) |