
 |
Mechanistic models of corrosion processes have been developed to incorporate a range of chemical and electrochemical reactions with the complex interactions of diffusion and migration details. Some specific situations lend themselves to the development of useful mechanistic models to account for the main features governing corrosion processes.
These models are most naturally expressed in terms of differential equations or in other non-explicit forms of mathematics.However, modern developments in computing facilities and in mathematical theories of non-linear and chaotic behaviors have made it possible to cope with relatively complex problems.A mechanistic model has the following advantages:
It contributes to our understanding of the phenomenon under study;
It usually provides a better basis for extrapolation;
It tends to be parsimonious, i.e. frugal, in the use of parameters and to provide better estimates of the response.
Rates of general corrosion in aqueous environments depend on a multitude of factors such as the chemistry of the aqueous solution, concentrations of components, temperature, presence of non-aqueous phases, hydrodynamic conditions and metallurgical factors. Therefore, it is desirable to rationalize and predict the effects of these factors using mechanistic models. The first comprehensive model that summarized many of the basic variables underlying a corrosion situation became known as potential-pH (E-pH) diagrams, also called predominance or Pourbaix diagrams, which have been adopted universally since their introduction in late 1940's.
These diagrams have repetitively been proven an elegant way to represent the thermodynamic stability of chemical species in given aqueous environments.E-pH diagrams are typically plotted for various equilibria on normal Cartesian coordinates with potential (E) as the ordinate (Y axis) and pH as the abscissa (X axis).
Pourbaix diagrams have been proven as a convenient way to summarize a situation in terms of its thermodynamics and provide a useful means of predicting electrochemical and chemical processes that could potentially occur in certain conditions of pressure, temperature, and chemical make-up. The following example illustrates potential corrosion damage to steel in water flowing through the radiators and pipes of a hydronic system. Given a pH range for mains water of 6.5 to 8 and the E-pH diagram in the following Figure, it is apparent that minimal corrosion damage is to be expected if the corrosion potential remains below -0.65V (SHE).
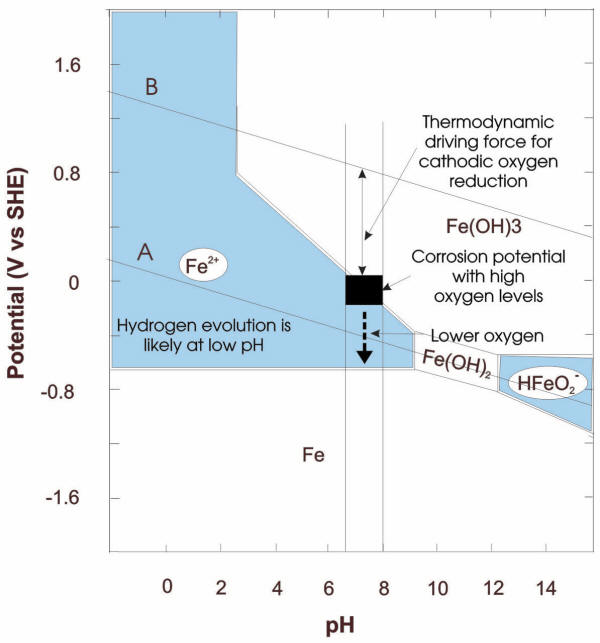
While E-pH diagrams were initially designed for relatively simple situations involving pure metals and pure water at a single temperature, the availability of computing power and basic thermodynamic information has permitted the development of these diagrams for increasingly complex situations, such as interaction associated with multi-element systems.
The use of polarization curves for the study of corrosion reactions can be traced back to the 1930's with the work of Wagner and Traud. However the representation of the mixed potential behavior is often associated with Professor Evans who has popularized this representation of corrosion polarization measurements.
See also: Boundary element modeling,Corrosion models, Knowledge based models, Mechanistic models, Pitting fatigue models, Risk based models