When hydrogen
ions are reduced to their atomic form they often combine, as shown earlier, to produce
hydrogen gas through reaction with electrons at a cathodic surface. This reduction
of hydrogen ions at a cathodic surface will disturb the balance between the acidic
hydrogen (H+) ions and the alkaline hydroxyl (
In
neutral waters
the anodic corrosion of some metals like aluminum, zinc, or magnesium, develops
enough energy to split water directly as illustrated in the following Figure and
equation.
![]()
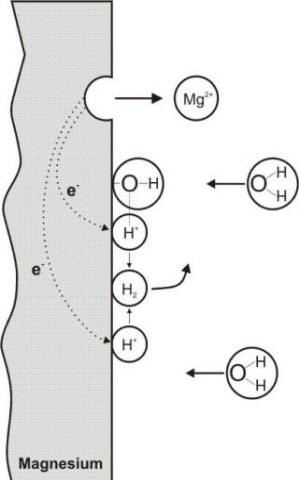
Electrochemical reactions occurring during the corrosion of magnesium in neutral water
The change
in the concentration of hydrogen ions or increase in hydroxyl ions can be shown
by the use of pH indicators which change color and thus can serve to demonstrate
and locate the existence of surfaces on which the cathodic reactions in corrosion
are taking place. There are several other cathodic reactions encountered during
the corrosion of metals. These are listed below.
![]()
![]()
![]()
![]()
![]()
Hydrogen ion
reduction, or hydrogen evolution, has already been discussed. This is the cathodic
reaction that occurs during corrosion in acids. Oxygen reduction is a very common
cathodic reaction, since oxygen is present in the atmosphere and in solutions exposed
to the atmosphere. Although less common, metal ion reduction and metal deposition,
can cause severe corrosion problems in special situations. One particular case worth
mentioning here is the plating of copper ions, produced upstream in a water circuit,
on the internal aluminum surface of a radiator, for example. The plated nodules,
which may form even at even at very low concentrations of copper ions, tend to be
dispersed and are thus a good catalyst for the subsequent reduction of dissolved
oxygen. It is therefore highly recommended to avoid using copper tubing in a water
circuit where aluminum is also present.
Such deposition
corrosion can be avoided by preventing the pick-up of cathodic ions that will enter
the equipment, or by scavenging them by passing the contaminated product through
a tower packed with more anodic metal turnings on which the ions can deposit.
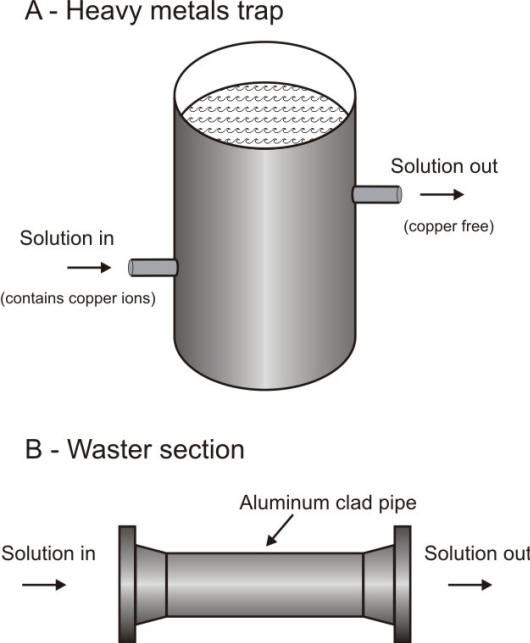
Method for removing troublesome ions from solution. a) Heavy metal trap: solutions containing copper ions enter barrel filled with aluminum shavings; b) Waster section: aluminum clad pipe inserted in a system removes heavy metal ions. The section is replaced once corroded.
Note that
all of the above reactions are similar in one respect; they consume electrons. All
corrosion reactions are simply combinations of one or more of the above cathodic
reactions, together with an anodic reaction. Thus, almost every case of aqueous
corrosion can be reduced to these equations, either singly or in combination.
Consider the
corrosion of zinc by water or moist air. By multiplying the zinc oxidation reaction
by 2 and summing this with the oxygen reduction reaction, one obtains the following
equation.
![]()
![]()
![]()
The products
of this reaction are Zn2+ and
![]()
![]()
![]()
During corrosion, more than one oxidation and one reduction reaction may occur. For example, during the corrosion of an alloy, its component metal atoms go into solution as their respective ions. Thus, during the corrosion of a chromium-iron alloy, both chromium and iron are oxidized. Also, more than one cathodic reaction can occur on the surface of a metal.
Consider the corrosion of zinc in a hydrochloric acid solution containing
dissolved oxygen. Two cathodic reactions are possible: the evolution of hydrogen
and the reduction of oxygen. Since there are two cathodic reactions or processes
which consume electrons, the overall corrosion rate of zinc is increased. Thus,
acid solutions which either contain dissolved oxygen or are exposed to air are generally
more corrosive than air-free acids.
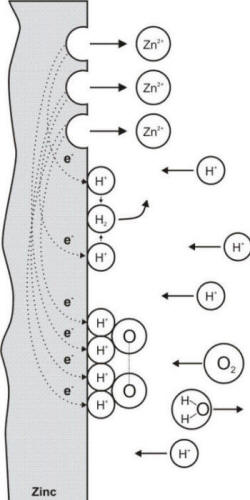
Electrochemical reactions occurring during the corrosion of zinc in aerated hydrochloric acid
Therefore, removing oxygen from acid solutions will often make these solutions less corrosive. This is a common method for reducing the corrosivity of many environments. Oxygen removal may be accomplished by either chemical or mechanical means.
If a piece of mild steel is placed in a solution of hydrochloric acid, a vigorous formation of hydrogen bubbles is observed. Under such conditions, the metal corrodes very quickly. The dissolution of the metal occurs only at anodic surfaces. The hydrogen bubbles form only at the cathodic surfaces, even though it may appear they come from the entire surface of the metal rather than at well-defined cathodic areas. The anodic and cathodic areas may shift from time to time so as to give the appearance of uniform corrosion.
If this action
could be seen through a suitable microscope, many tiny anodic and cathodic areas
would be observed shifting around on the surface of the metal. These areas, however,
are often so small as to be invisible and so numerous as to be almost inseparable.
| (previous) | Page 5 of 6 |
|