
 |
When a piece of metal is freely corroding, the electrons generated at anodic areas flow through the metal to react at cathodic areas similarly exposed to the environment where they restore the electrical balance of the system. The fact that there is no net accumulation of charges on a corroding surface is quite important for understanding most corrosion processes and ways to mitigate them. However, the absolute equality between the anodic and cathodic currents expressed in the following equation does not mean that the current densities for these currents are equal.
![]()
When this equation is expressed in terms of current densities by considering the relative anodic (Sa) and cathodic (Sc) surface areas and their associated current densities ia and ic expressed in units of mA/cm2, for example, it becomes clear that a difference in the surface areas occupied by each reaction will have to be compensated by inequalities in the current densities.
![]()
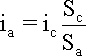
The implications of the surface area ratio Sc/Sa in the last equation are particularly important in association with various forms of local cell corrosion such as pitting and stress corrosion cracking for which a large surface area ratio is a serious aggravating factor. It is easy to understand that the effect of a certain amount of anodic current concentrated on a small area of metal surface will be much greater than when the effect of the same amount of current is dissipated over a much larger area. This factor is an important amplifying factor of the anodic current when Sc/Sc is >> 1 and a stifling factor when it is << 1.
This area effect in terms of current density is illustrated by combinations of steel and copper as either plates or the fasteners used to join them and immersed in a corrosive solution. If steel rivets are used to join copper plates, the current density on the relatively large cathodic copper plates will be low, cathodic polarization of the copper will be slight, and the voltage of the galvanic couple will maintain a value close to the open circuit potential. At the same time, the current density on the small anodic steel rivets will be high and the consequent corrosion quite severe, giving rise to a particularly vicious form of corrosion called galvanic corrosion.
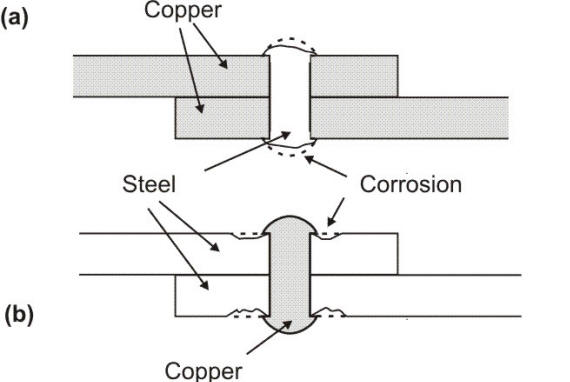
Galvanic coupling caused by riveting with dissimilar metals: a) steel rivets on copper plates, b) copper rivets on steel plates
With the opposite arrangement of copper rivets joining steel plates, the current density on the copper cathodes will be high, with consequently considerable cathodic polarization of the copper reducing the open circuit potential below its initial value. The diminished anodic current will be spread over the relatively large steel plates and the undesirable galvanic effect will hardly be noticeable.
Open circuit potential measurements are grossly inadequate for predicting the magnitude of galvanic effects since they do not take into account area and polarization effects. They are reliable only for predicting the direction of such effects.
| (previous) | Page 6 of 6 | (next Module) |