
 |
These are the types of corrosion in which there is intense attack at localized sites on the surface of a component whilst the rest of the surface is corroding at a much lower rate - either because of an inherent property of the component material (such as the formation of a protective oxide film) or because of some environmental effect. Indeed the main surface may be essentially under satisfactory corrosion control. In such circumstances, if corrosion protection breaks down locally then corrosion may be initiated at these local sites. If this event occurs under a deposit on the surface (perhaps a weld deposit or some solid debris from the environment) or at the joint of a bolted assembly etc, the attack is termed, “crevice corrosion”. If the attack initiates on the free surface of a component, it is termed “pitting”. The resistance to these two types of localized corrosion varies greatly between different materials and is extremely dependent upon environmental factors.
The occurrence of localized corrosion is a manifest proof that the anodic surface area can be much smaller than the cathodic. The Sa/Sc ratio, or degree of localization, can be an important driving force of all localized corrosion problems since a corrosion situation corresponds to equal anodic and cathodic absolutes currents. Corrosive microenvironments, which tend to be very different from the bulk environment, often play a role in the initiation and propagation of corrosion pits. This greatly complicates the prediction task.
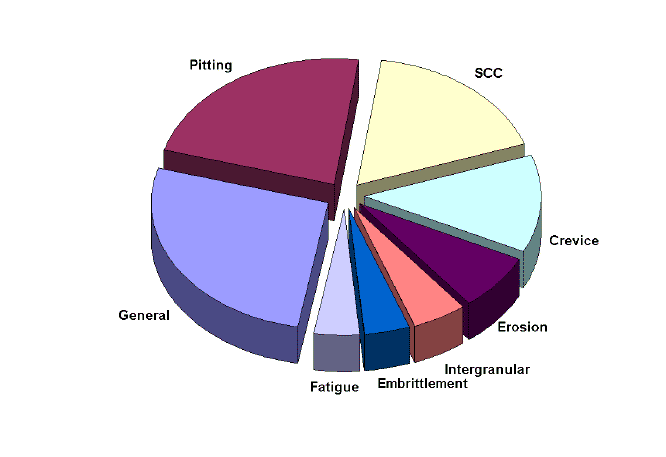
In general terms, small corrosion anodic areas correspond to severe corrosion problems with low detectability. The industrial importance of localized corrosion problems has been revealed in many reports. The following pie chart summarizes the findings of 363 corrosion failure cases investigated in a major chemical processing company[1]. The importance of pitting comes second (22%) after general corrosion and before stress corrosion cracking (SCC) which is, by the way, often initiated by pitting. Crevice corrosion comes fourth at 12%.
| (previous) | Page 3 of 23 | (next) |
[1]F. Dabosi, G. Béranger and B. Baroux, Corrosion Localisée, les éditions de physique, France, 1994, p. 654.