Crevice Corrosion |
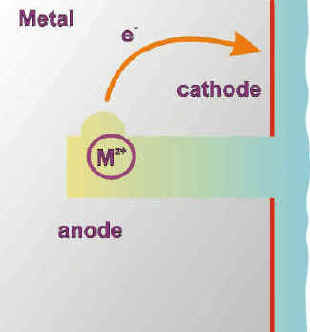
Because of the difficult access caused by the crevice geometry, oxygen consumed by normal uniform corrosion is very soon depleted in the crevice. The corrosion reactions now specialize in the crevice (anodic) and on the open surface (cathodic). The large cathodic surface (Sc) vs. anodic surface (Sa) ratio (Sc/Sa) that forms in these conditions is a definitive aggravating factor of the anodic (corrosion) reaction.
Assuming the total surface area is still 100 cm2 with 99 cm2 occupied by the cathodic area and only 1 cm2 corresponding to the crevice or anodic surface. In reality this imbalance can be larger or smaller depending on local geometry characteristics.
The surface area ratio would then be 99/1 or 99.
Assuming the initial current density of 0.022 mA cm-2 is the same for the cathodic reaction this would mean that the total cathodic current would be equal to 99 cm2 x 0.022 mA cm-2 or 2.18 mA.
This also mean that the corrosion or anodic reaction has to provide the electrons to satisfy the cathodic demand (see corrosion theory and mixed potential diagram for more details)
The total anodic current would therefore now be 2.18 mA and the anodic current density be 2.18 mA/1cm2 or 2.18 mA cm-2.
Consulting the conversion table we find that this current density now corresponds to a corrosion penetration rate of 25 mm y-1 or 990 mpy for the same steel specimen.
This oversimplified example illustrates one aspect of a crevice situation. The next step in a crevice situation stabilizes it into a permanent problem that will need drastic corrective measures.
| (previous) | Page 7 of 23 | (next) |