Crevice Corrosion |
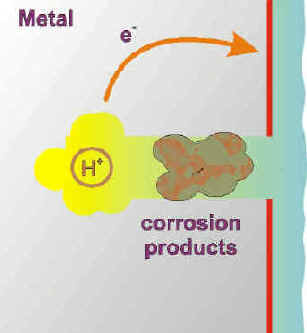
The metal ions produced by the anodic corrosion reaction readily hydrolyze giving off protons (acid) and forming corrosion products. The pH in a crevice can reach very acidic values, sometimes equivalent to pure acids.
The acidification of the local environment can produce a serious increase in the corrosion rate of most metals. See, for example, how the corrosion of steel is affected as a function of water pH.
The corrosion products seal even further the crevice environment.
The accumulation of positive charge in the crevice becomes a strong attractor to negative ions in the environment, such as chlorides and sulfates, that can be corrosive in their own right.
| (previous) | Page 8 of 23 | (next) |