Nickel Allergy
As Be Cd Cr Co Cu F Pb Hg Ni Tl
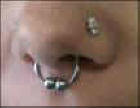 Contact allergic dermatitis to nickel may develop at any age. Once this nickel
allergy has occurred, it persists for many years, often it will remain for the
rest of your life. Nickel allergy is more common in women, probably because they are more likely
to have pierced ears than men, although this
is changing.
Contact allergic dermatitis to nickel may develop at any age. Once this nickel
allergy has occurred, it persists for many years, often it will remain for the
rest of your life. Nickel allergy is more common in women, probably because they are more likely
to have pierced ears than men, although this
is changing.
Allergy to nickel is a phenomenon which has assumed growing importance in recent years, largely because of the introduction of cheap fancy jewellery in which the underlying metal layer consists of nickel. 10 to 12% of the female population and 6% of the male population are estimated to be allergic to nickel. In fact the allergy is not caused by nickel itself but by the nickel salts which are formed under the effect of perspiration in contact with the piece of jewellery piece or watch. This phenomenon is always accompanied by corrosion of the object. (reference)
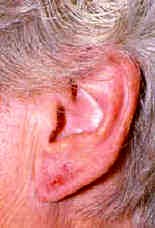
degree of allergy varies. Some people develop dermatitis (also called eczema) from even brief contact with nickel-containing items, while others break out only after many years of skin contact with nickel. Some people develop intermittent or persistent eczema on their hands and feet. It is usually a blistering type of eczema, known as pompholyx. Sometimes it is due to contact with metal items containing nickel, but often there is no obvious reason for it. It has been suggested that in some, dyshidrotic hand dermatitis is due to nickel in the diet. Unfortunately it is not possible to avoid ingesting nickel as it is present in most foodstuffs. A low-nickel diet is only rarely helpful.
If you suffer from this type of allergy you should avoid contact with nickel-containing metals. Test your metal items to see if they contain nickel. Obtain a nickel-testing kit from your dermatologist or pharmacist. The kit consists of two small bottles of clear fluid. One contains dimethylglyoxime and the other ammonium hydroxide. When mixed together in the presence of nickel, a pink color results. Apply a drop from each bottle on to the metal item to be tested, first try it on a 10 cent coin. Use a cotton bud to rub gently - observe the color on the bud. If it remains clear, the item has no free nickel and will not cause dermatitis. If it is pink it contains nickel and may cause problems if the metal touches your skin. The chemicals will not harm your jewelry.
- Jewelry: Necklaces, necklace-clips, earrings, bracelets, watch-straps and rings may contain nickel. "Hypoallergenic", solid gold (12 carat or more) and silver jewellery should be safe. Nine carat gold and white gold both contain nickel. Plastic covers for earring studs can be obtained. Coating the stud with nail varnish is not recommended.
- Clothing: Metal zips, bra hooks, suspender clips, hair-pins, buttons, studs, spectacle frames etc. are likely to contain nickel. Use substitutes made of plastic, coated or painted metal or some other material.
- Personal articles: Consider lipstick holders, powder compacts, handbag catches, cigarette lighters, razors, keys, key rings, pocket knives, pens as potential causes of dermatitis.
- Metal items in the home: Cupboard handles, kitchen utensils, cutlery, toaster, metal teapots, scissors, needles, pins, thimble, vacuum cleaners, torches, bath plugs... may all contain nickel. Choose tools with plastic handles. Stainless steel does not usually cause dermatitis unless it is nickel-plated.
- Money: Silver coins are composed of cupro-nickel. Cashiers with nickel allergy may develop hand dermatitis from this source. Wear gloves to handle money or pay via credit card machines or with a check.
- Metal at work: Nickel dermatitis may be aggravated by contact with paper clips, typewriter keys, instruments, metal fragments from a lathe or chain saw.
Gold Allergy ?
An allergy is a hypersensitivity to a substance that causes the body to react
to any contact with that substance. Do you have an allergy to gold? Probably
not, that is not gold itself. What people occasionally have is an allergy to
the nickel content in gold alloys, usually in the alloy rich 9ct gold. 9ct alloys
go dull or even black from the exposure to chemicals in the atmosphere and also
may discolor from contact with perspiration, bleach, household chemicals and
some fabrics. (reference)
Allergenic Effects of Metals and Dental Casting Alloys
Metal ions, which are released from dental restorations, can provoke systemic and local allergic reactions. A case has been reported of an individual who suffered from an IgA nephropathy after placement of nickel alloy base crowns. An analysis of 139 published cases of allergic reactions to dental metallic restorations showed that those patients suffered from local irritations predominantly in the form of gingivitis and stomatitis. (reference)
Only 33 out of 139 persons revealed general reactions. Analysis of these data demonstrates that local allergic reactions to metals may often be misdiagnosed as inflammatory reactions. Most frequently, lichenoid reactions and swelling and pain of oral soft tissues and lips were observed. In several patients, Au ions caused an allergic contact gingivo-stomatitis which was similar in appearance to an erosive lichen planus. Interestingly, many patients had a history of intolerance to gold jewelry. Lichenoid reactions of the oral mucosa have also been found when Ni was used in an intra-oral patch test.
A double-blind clinical study including a patch-test screening of 708 patients showed 12 positive results (1.7%): Ni (five), Cr (six), and Co (one) (Morris, 1987). But it should be noted that positive skin reactions to metals such as Ni are not necessarily associated with intra-oral allergic reactions. For instance, it was observed that patients with a previous history of positive skin reactions to Ni did not develop local or systemic adverse effects in response to a Ni-containing alloy during an observation period of up to 15 years.
No association between patients' orofacial complaints and patch test results was found. This clearly indicates that routine patch-testing of patients with subjective intra-oral symptoms should not be performed. It is also noteworthy that patch tests may even cause sensitization in some cases. Various "porcelain fused" casting alloys contain iridium (Ir) and indium (In). Positive patch-test reactions to these metals were observed in five out of 205 patients investigated.
A study with 60 persons documented that nickel has the highest allergenic potency, followed by K, Co, Ag, Cu, Pd, Pt, and Au. 86 patients were examined who suffered from adverse intra-oral effects due to cast metallic restorations. Twenty-three percent of these persons exhibited gingivitis which did not disappear after thorough plaque control. Alterations of the tongue were observed in 16% of the patients, and five out of 86 persons demonstrated oral lichenoid lesions.
Allergic reactions to 49 selected casting alloys, commercially pure titanium (cpTi), and amalgam were investigated in an epidemiological study population of 763 patients. Most frequently, Ni, Co, and Ag caused allergic irritations. In addition, a surprisingly high incidence of positive dermal reactions to Ti was observed. Analysis of these data indicates that Ti should also be considered in the diagnosis of allergic alterations due to metallic dental restorations. Contact sensitivity to a Ti device (pacemaker) has also been reported.
Metals of the platinum group exhibit a considerable allergenic potency. In particular, allergic reactions to palladium. e.g. such as contact dermatitis, intra-oral contact mucositis, and lichenoid reactions, have been reported. Interestingly, from 34% to 64.5% of patients who are allergic to Ni are also allergic to Pd ions. Further, Pd sensitivity always occurs with Ni allergy. But only a few of these patients will also react to the metal form of Pd.
A high sensitization rate to Pd (8.3%) was found in randomly selected eczema patients. Therefore, the authors questioned the "clinical future" of Pd-Ag alloys. In contrast it has also been stated that Pd, as a component of dental casting alloys, does not pose an increased risk to the health of patients, since the dissolution rate of Pd ions from these alloys is very low.
Gold restorations significantly increase the prevalence of gold sensitivity. One hundred thirty-six asymptomatic patients were patch-tested for gold sensitivity. Of these, 33.8% of persons with gold restorations had positive skin reactions, whereas only 10.8% of the patients without gold restorations revealed positive reactions.
A test battery has been created for the detection of allergic reactions to metal ions released from dental restorations. The most important cations concerning corrosion and related allergy were used for this test battery and included Au3+, Pd2+, Zn2+, Mo6+, Sn2+, Ga3+, Co2+, Cr3+, Cr6+, Ni2+, Fe2+, Fe3+, and Si4+. Subsequently, the relevance of those cations for the detection of allergic reactions to dental metallic restorations was investigated in patients with a history of contact stomatitis, contact dermatitis, and healthy control persons. Patch tests revealed a significantly higher percentage of positive reactions in allergy patients in comparison with healthy control persons. The most relevant cations were Pd and Ni, whereas Ga, Sn, and Zn caused no adverse reactions.
Although allergies to dental castings are not very frequent, a suitable alloy should be selected only after careful evaluation of its allergenic or immunological consequences. This was corroborated during an investigation of the prevalence of sensitivity to amalgam and casting alloys in 95 randomly selected persons. Six individuals developed positive reactions in epicutaneous patch tests to constituents of cast alloys, such as Cu, Ni, Co, Au, and Zn. In addition, medical devices (pacemakers), jewelry, and dental metallic restorations may cause metal allergy in patients.
Ni, in particular, has been shown to be highly allergenic. Therefore, Ni-containing alloys should be avoided in persons with a history of Ni allergy. But there is a lack of agreement if the application of Pd alloys significantly increases the risk of allergic reactions to this ion. It must be emphasized, however, that many patients (from 34% to 65.5%) who are allergic to Ni are also allergic to Pd. This aspect should be taken into serious consideration before the use of Pd-containing alloys in patients who are allergic to Ni.
Toxic Elements: Arsenic, Beryllium, Cadmium, Chromium, Cobalt, Copper, Fluorine, Lead, Mercury, Nickel, Thallium
