Rusting of Metals
|
Developers: |
Nick Timpanelli Woodrow Wilson High Camden, NJ |
Dr. Craig Allen Rohm and Haas Company Bristol, PA |
|
Grade Levels: |
High School |
|
Objectives: |
Students will be able to observe and record the corrosive nature of oxidation-reduction reactions and to determine the electro-chemical series of selected metals (relative strengths of oxidizing and reducing agents). |
Introduction:

Rusting of metals is a special case of metal oxidation. Iron will oxidize to form
rust.* Water will cause metals to rust; this reaction can be accelerated by adding
salts. In the corrosion process, metals get oxidized. For example in mild steel
(which is greater than 99% iron) the metal corrodes according to the following:
![]()
![]()
![]()
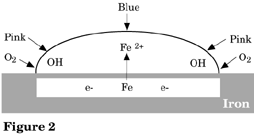
These equations indicate that in order for metals to corrode (rust), two reactions occur; an oxidation that converts metal to metal ions and electrons and a second reaction which consumes those electrons by converting oxygen and water to hydroxide ions. In order for these reactions to occur, the electrons must be transported from the place where the metal dissolves to the place where the oxygen is consumed and an ionic current must also flow between the sites to complete the circuit. This ionic current flows more easily through water containing electrolytes (i.e., NaCl). This accounts for the rapid rusting of unprotected steel in a salty environment.
The final product of iron oxidation (rust) is usually a ferric oxide (often hematite Fe2O3). The initial corrosion product of the anodic reaction is ferrous (Fe2+) ion. This is subsequently oxidized to Fe3+ by exposure to oxygen. In this experiment we are looking at the initial product only.
In this experiment we can watch the corrosion reaction by using substances that produce a color change when they react with the products of the iron oxidation or oxygen reduction. Recall that phenolphthalein turns pink in the presence of hydroxide and ferricyanide turns a deep blue in the presence of iron II++ (rust). (see experiment #6, Metcalfe, H. Clark, Modern Chemistry, Holt, Reinhart and Winston, 1982, pp. 147-50.
The corrosion process may be slowed by coating the metals with other metals or polymers in order to protect the metal from the corrosive environment. Examples of this can be seen in food cans which have a polymer coating and in galvanized steel where iron is coated with zinc.
When we put two metals in direct contact, one can oxidize (rust) while the other reduces oxygen. This reaction sets up a voltage and is the primary reaction in a battery. By measuring this voltage, it is possible to construct a list ranking the metal's oxidation tendencies. If metals which are far apart in oxidation tendencies are placed in contact with each other and with an electrolyte solution, severe corrosion of one metal can occur. We will examine some of these metal combinations in this experiment.
Apparatus:
Watch glass; dropper; fine sandpaper; digital volt meter; 100-ml wide-mouth bottle; meter leads with alligator clips; rubber stopper, #8 with a #12 hole bored; triangular file.Materials:
0.1 M K4Fe(CN)6; 0.1 M K3Fe(CN)6: 0.1 M NaCl solution: phenolphthalein; Al, Cu, Pb, Sn, Zn, 1.5 X 6 cm foil strips; Mg ribbon, 6 cm; mild steel bar 2.5 X 6 cm; galvanized steel sheet, 4 X 4 cm; steel can (food) lids, polymer coated; steel can side, tin coated (use Hunt's tomato sauce can); pennies, new and old (pre-1982).
Safety Note:
Wear safety goggles, aprons and gloves. No open flame should be present in the room. Do not use acid during any part of this lab due to the danger of generating cyanide gas. Read all containers before use. Tape metal edges.Part I:
Rusting of Steel Using the Salt Drop Technique. (First described in 1926 by U. R. Evans. See Scully, J. C., The Fundamentals of Corrosion, 2nd Ed., Pergamon. 1975. p. 57.)
Procedures:
1. Plain Steel
Obtain 100 ml of salt solution and add 10 drops of phenolphthalein. On a section of mild steel, combine 4 drops of this solution and 3 drops of potassium ferricyanide and cover with a watchglass. Observe for at least five minutes. What changes occur?
On the same bar do as above except use ferrocyanide. Observe for at least 5 minutes. What changes occur? Which chemical reagent (ferro or ferri) would you use to check for rust on iron?
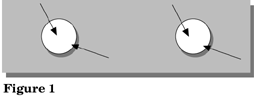
In Figure 1, fill in the colors you see at the proper sites.
Ions are spatially separated in this salt drop experiment because the drop is thicker in the middle than at the edges. Electrochemical reduction reactions that produce OH- occur at the edges due to readily available oxygen from the air. Electrochemical oxidation reactions occur at the middle of the drop due to the lack of oxygen. See Figure 2.
2. Polymer Coated Steel
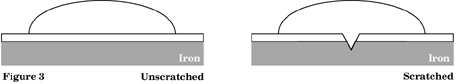
Using the file, place a deep scratch on one area of a polymer coated steel can lid. Place 3 drops of ferricyanide and 4 drops of salt solution on the scratch. On a second area of the polymer coated lid, place the drops as above and cover with a watch glass. Observe both areas of the lid for at least 5 minutes. What changes occur? Record in Figure 3.
3. Tin Coated Steel
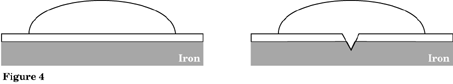
Repeat Procedure 2 using a tin plated steel can side, tin side up. Observe for at least 8 minutes. What changes occur? Record in Figure 4.
4. Zinc Plated Steel
Repeat Procedure 2 using a piece of galvanized steel. Observe for 5 minutes. What changes occur? Is iron rusting? Record in Figure 5.
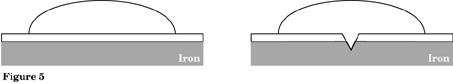
Unscratched__________________ Scratched ___________________
Observe that an intense pink color forms, indicating a reaction is taking place and OH ions are produced. No blue is seen in the drop-indicating that the iron is not rusting. Metals such as zinc are used because these sacrificial anodes are more willing to give up electrons (oxidize) than the iron and thus protect the iron from oxidation. Let us investigate different metal combinations.
3. A Penny For Your Thoughts
Following Procedures 2 in Part I. use the salt drop technique on each of two pennies with a deep scratch on each (one penny pre-1982 and one post-1982). The new pennies are copper plated zinc. What do you think will happen?
Part II - Galvanic Series (batteries)
Procedure:
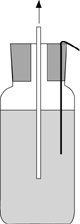
1. Voltmeter Ranking of Metals
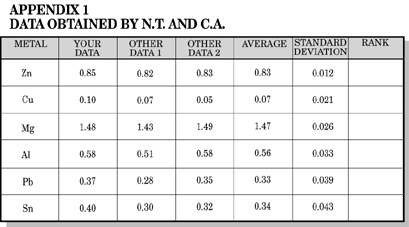
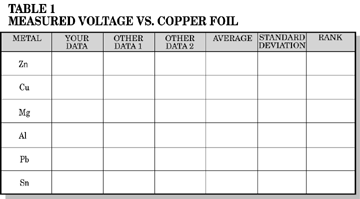
Fill a wide mouth bottle with salt solution. Hang a copper strip over the side of the jar, and stopper the jar. Abrade all metal strips with sandpaper. Clip one lead from a voltmeter to the copper strip and the second lead to a metal strip into the solution through the hole in your stopper and record the voltage on Table 1. Obtain two more sets of readings from other students; average and calculate the standard deviation. Rank your metals in ascending order of voltage.
|
|
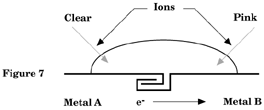
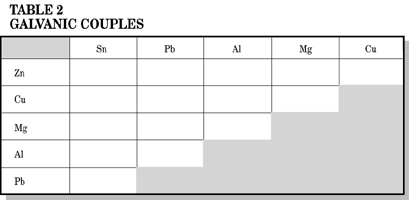
2. Galvanic Couples of Metals
Place 2 strips of metal from Table 2 on each other and fold one end of the strips over each other several times. Flip one metal out so that both metals are visible (see figure 7). Place several drops of your salt solution on the junction of the 2 metals. Observe and record which metal turns pink on Table 2. In using Mg, if both metals turn pink, ignore the Mg.
metal A —e--—> metal B
The metal acting as a cathode turns pink therefore the other metal must be the anode and is corroding (rusting). How do the results in Table 2 compare with the voltage ranking on Table 1?
Questions:
- Explain your observations and conclusions from the pennies experiment above.
- Why does grapefruit juice left in an open can taste metallic?
- If nerves respond to electrical currents, why do you think putting aluminum foil on an amalgam (gray) filled tooth hurts? Dental amalgam is a mixture of Ag, Sn, and Hg.
- Why do they put magnesium rods in a steel hot water heater? (Hint: Think about galvanized steel.)
- If pipes feeding a water fountain were made of copper with lead solder at the junctions, which metal dissolves more readily? Explain.
- Tarnished silver can be restored by contact with magnesium in a salt solution. In this reaction, the tarnished silver is reduced. What is oxidized? (Try this!)

Connect with us
Contact us today