
 |
Electroplating is achieved by passing an electrical current through a solution containing dissolved metal ions and the metal object to be plated. The metal object serves as the cathode in an electrochemical cell, attracting metal ions from the solution. Ferrous and non-ferrous metal objects are plated with a variety of metals, including aluminum, brass, bronze, cadmium, copper, chromium, iron, lead, nickel, tin, and zinc, as well as precious metals, such as gold, platinum, and silver. The process is regulated by controlling a variety of parameters, including the voltage and amperage, temperature, residence times, and the purity of bath solutions.
For some metals all these demands can not be fulfilled using a water based electrolyte. Elements such as Ti and Al can only be deposited from organic electrolytes, while other metals such as Mg, Nb, Ta, and W can only be plated from molten salt electrolytes (at 700°C and above).
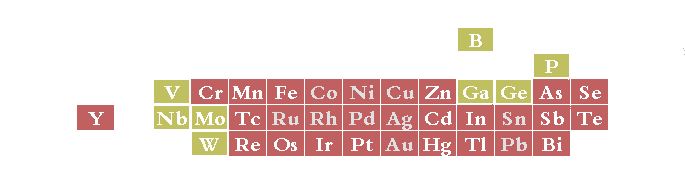
Metals plateable from aqueous solutions (red background). Elements with yellow background are only plateable in combination with one of the others (alloy plating). Grey text indicates that the element can be deposited both by an auto-catalytic electroless process or by electroplating. (reference 81)
The sequence of unit operations in an electroplating operation typically involves various cleaning steps, stripping of old plating or paint, electroplating steps, and rinsing between and after each of these operations. Electroless plating uses similar steps but involves the deposition of metal on a substrate without the use of external electrical energy.
See also: Cladding, Electroplating, Pack cementation, Electroless plating, Vapor deposition, Hot dip galvanizing, Thermal spraying, Zinc coatings