Periodic Table of the Elements
The modern periodic table has the elements arranged in order of increasing atomic number. A vertical column, called a group, contains elements with similar properties. A period runs from left to right with the atomic number of the elements increasing from left to right.
A periodic table showing the currently recommended International Union of Pure and Applied Chemistry (IUPAC) group numbering system
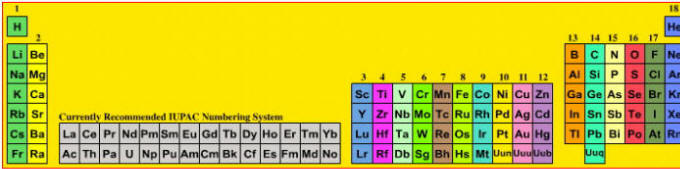
A periodic table showing the previously recommended IUPAC group numbering system.

Discovery of the elements
Notice how many elements were discovered in the 19th century. In 1800, only a couple of dozen elements where known. By 1900, the list was more or less complete (as far as the non-radioactive elements are concerned).
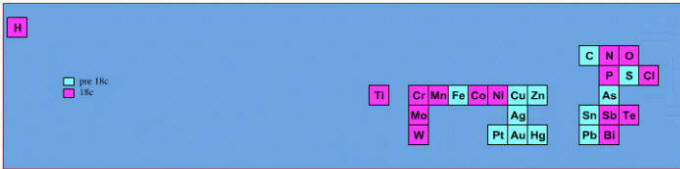
Elements known before 1700:

Elements known before 1700, plus those discovered between 1700 and 1799:
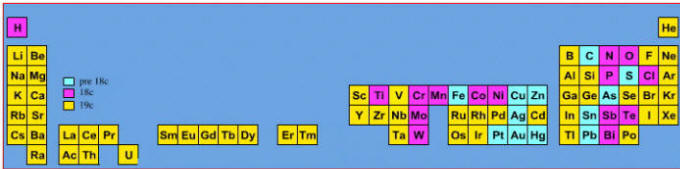
Elements known before 1800, plus those discovered between 1800 and 1899:
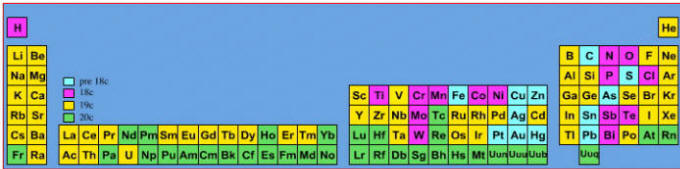
Elements known before 1900, plus those discovered between 1900 and 2005:
Development of the Periodic Table
Before the development of the modern periodic table, there were other attempts to arrange the elements in a useful way. For example, Dobereiner arranged groups of three elements with similar properties into 'triads'. Newlands and Mendeleev arranged the elements in order of increasing relative atomic mass.
Some attempts at arranging the elements were more successful than others. Newlands did not leave any space for undiscovered elements but Mendeleev did. Using his table, Mendeleev was able to successfully predict the properties of three elements that had not yet been discovered. The final modern periodic table arrangement was finally proposed by Moseley in 1913 by ordering elements by their atomic number instead of their mass.
In 1944, Seaborg formulated the 'actinide concept' of heavy element electronic structure which predicted that the actinides – including the first eleven transuranium elements – would form a transition series analogous to the rare earth series of lanthanide elements. Called one of the most significant changes in the periodic table since Mendeleev's 19th century design, the actinide concept showed how the transuranium elements fit into the periodic table.
Resources
See also: Development of the Periodic Table, de Chancourtois, Dobereiner, Dumas, Mendeleev, Meyer, Moseley, Newlands, Seaborg

Connect with us
Contact us today