Newlands' Periodic Table

John Alexander Reina Newlands (November 26, 1837 - July 29, 1898, London)
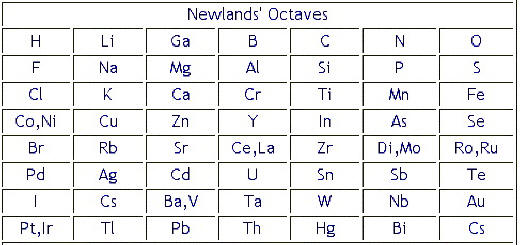
Newlands studied at the Royal College of Chemistry and worked as an analytical chemist. Continuing the work of Johann Wolfgang Döbereiner with triads and the work of Jean-Baptiste Dumas families of similar elements, he published in 1865 his 'Law of Octaves', an innovative concept proposing the periodicity of the chemical elements arranged in order of atomic weight. He pointed out that every eighth element in this grouping shared a resemblance and suggested an analogy with the intervals of the musical scale. John Newlands put forward his law of octaves in 1864 in which he arranged all the elements known at the time into a table in order of relative atomic mass. When he did this, he found that each element was similar to the element eight places further on. For example, starting at Li, Be is the second element, B is the third and Na is the eighth element.
The incompleteness of the table alluded to the possible existence of additional, undiscovered elements. However, the Law of Octaves was ridiculed by some of Newlands' contemporaries, and the Society of Chemists did not accept his work for publication.
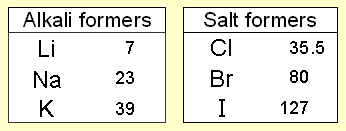
Regular repeats Newlands' table showed a repeating or periodic pattern of properties, but it had problems. For example, he put iron in the same group as oxygen and sulphur, which are two non-metals. As a result, his table was not accepted by other scientists. After Dmitri Mendeleev and Lothar Meyer received the Davy Medal from the Royal Society for their later 'discovery' of the periodic table, Newlands fought for recognition of his earlier work and eventually received the Davy Medal in 1887.
See also: Development of the Periodic Table, de Chancourtois, Dobereiner, Mendeleev, Moseley, Newlands, Seaborg

Connect with us
Contact us today