 |
|
 |
Indestructibility of Matter
in Chapter 1 of Principles of Chemistry
All the progress made by chemistry during the end of the last, and in the present, century is entirely and immovably founded on the law of the indestructibility of matter. It is absolutely necessary in beginning the study of chemistry to become familiar with the simple truth which is expressed by this law, and for this purpose several examples elucidating its application will now be cited.
- It is well known that iron rusts in damp air,' and that when heated to redness in air it becomes coated with scoria (oxide), having, like rust, the appearance of an earthy substance resembling some of the iron ores from which metallic iron is extracted. If the iron is weighed before and after the formation of the scoria or rust, it will be found that the metal has increased in weight during the operation. It can easily be proved that this increase in weight is accomplished at the expense of the atmosphere, and mainly, as Lavoisier proved, at the expense of that portion which is called oxygen. In fact, in a vacuum, or in gases which do not contain oxygen, for instance, in hydrogen or nitrogen, the iron neither rusts nor becomes coated with scoria. Had the iron not been weighed, the participation of the oxygen of the atmosphere in its transformation into an earthy substance might have easily passed unnoticed, as was formerly the case, when phenomena like the above were, for this. reason, misunderstood. It is evident from the law of the indestructibility of matter that as the iron increases in weight in its conversion into rust, the latter must be a more complex substance than the iron itself, and its formation is due to a reaction of combination. We might form an entirely wrong opinion about it, and might, for instance, consider rust to be a simpler substance than iron, and explain the formation of rust as the removal of something from the iron. Such, indeed, was the general opinion prior to Lavoisier, when it was held that iron contained a certain unknown substance called 'phlogiston,' and that rust was iron deprived of this supposed substance.
- Copper carbonate (in the form of a powder, or as the well-known green mineral called ' malachite,' which is used for making ornaments, or as an ore for the extraction of copper) changes into a black substance called 'copper oxide' when heated to redness. This black substance is also obtained by heating copper to redness in air that is, it is the scoria or oxidation product of copper. The weight of the black oxide of copper left is less than that of the copper carbonate originally taken, and therefore we consider the reaction which occurred to have been one of decomposition, and that by it something was separated from the green copper carbonate, and, in fact, by closing the orifice of the vessel in which the copper carbonate is heated with a well- fitting cork, through which a gas delivery tube passes whose end is immersed under water, it will be observed that en heating, a gas is formed which bubbles through the water. This gas can be easily collected, as will presently be described, and it will be found to essentially differ from air in many respects ; for instance, a burning taper is extinguished in it as if it had been plunged into water. If weighing had not proved to us that some substance had been separated, the formation of the gas might easily have escaped our notice, for it is colorless and transparent like air, and is therefore evolved without any striking feature. The carbonic anhydride evolved may be weighed, and it will be seen that the sum of the weights of the black copper oxide and carbonic anhydride is equal to the weight of the copper carbonate originally taken, and thus by carefully following out the various stages of all chemical reactions we arrive at a confirmation of the law of the indestructibility of matter.
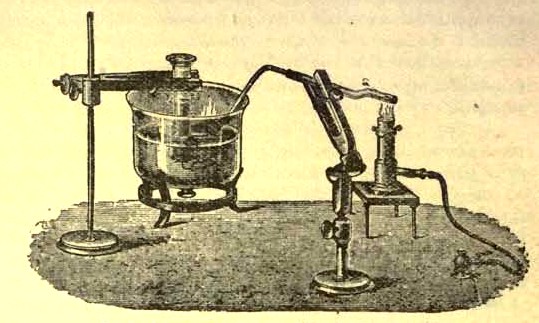
Figure 1 - Apparatus for the decomposition of red mercury oxide.
- Red mercury oxide (which is formed as mercury rust by heating mercury in air) is decomposed like copper carbonate (only by heating more slowly and at a somewhat higher temperature), with the formation of the peculiar gas, oxygen. For this purpose the mercury oxide is placed in a glass tube or retort,18 to which a gas delivery tube is attached by means of a cork. This tube is bent downwards, as shown in the drawing (Fig. 1). The open end of the gas delivery tube is immersed in a vessel filled with water, called a pneumatic trough. When the gas begins to be evolved in the retort it is obliged, having no other outlet, to escape through the gas delivery tube into the water in the pneumatic trough, and therefore its evolution will be rendered visible by the bubbles coming from this tube. In heating the retort containing the mercury oxide, the air contained in the apparatus is first partly expelled, owing to its expansion by heat, and then the peculiar gas called ' oxygen' is evolved, and may be easily collected as it comes off. For this purpose a vessel (an ordinary cylinder, as in the drawing) is filled quite full with water and its mouth closed ; it is then inverted and placed in this position under the water in the trough ; the mouth is then opened. The cylinder will remain full of water—that is, the water will remain at a higher level in it than in the surrounding vessel, owing to the atmospheric pressure.
The atmosphere presses on the surface of the water in the trough, and prevents the water from flowing out of the cylinder. The mouth of the cylinder is placed over the end of the gas delivery tube, 2e and the bubbles issuing from it will rise into the cylinder and displace the water contained in it. Gases are generally collected in this manner When a sufficient quantity of gas has accumulated in the cylinder it can be clearly shown that it is not air, but another gas which is distinguished by its capacity for vigorously supporting combustion In order to show this, the cylinder is closed, under water, and removed from the bath , its mouth is then turned upwards, and a smoldering taper plunged into it. As is well known, a smoldering taper will be extinguished in air, but in the gas which is given off from red mercury oxide it burns clearly and vigorously, showing the property possessed by this gas for supporting combustion more energetically than air, and thus enabling it to be distinguished from the latter. It may be observed in this experiment that, besides the formation of oxygen, metallic mercury is formed, which, volatilizing at the high temperature required for the reaction, condenses on the cooler parts of the retort as a mirror or in globules. Thus two substances, mercury and oxygen, are obtained by heating red mercury oxide. In this reaction, from one substance, two new substances are produced—that is, a decomposition has taken place. The means of collecting and investigating gases were known before Lavoisier's time, but he first showed the real part they played in the processes of many chemical changes which before bis era were either wrongly understood (as will be afterwards explained) or were not explained at all, but only observed in their superficial aspects.
his experiment on red mercury oxide has a special significance in the history of chemistry contemporary with Lavoisier, because the oxygen gas which is here evolved is contained in the atmosphere, and plays a most important part in nature, especially in the respiration of animals, in combustion in air, and in the formation of rusts or scoriae (earths, as they were then called) from metals that is, of earthy substances, like the ores from which metals are extracted. 4. In order to illustrate by experiment one more example of chemical change and the application of the law of the indestructibility of matter, we will consider the reaction between common table salt and lunar caustic, which is well known from its use in cauterizing wounds By taking a clear solution of each and mixing them together, it will at once be observed that a solid white substance is formed, which settles to the bottom of the vessel, and is insoluble in water. This substance may be separated from the solution by filtering , it is then found to be an entirely different substance from either of those taken originally in the solutions. This is at once evident from the fact that it does not dissolve in water On evaporating the liquid which passed through the filter, it will be found to contain a new substance unlike either table salt or lunar caustic, but, like them, soluble in water Thus table salt and lunar caustic, two substances soluble in water, produced, by their mutual chemical action, two new substances, one insoluble in water, and the other remaining in solution. Here, from two substances, two others are obtained, consequently there occurred a reaction of substitution. The water served only to convert the re-acting substances into a liquid and mobile state. If the lunar caustic and salt be dried Si and weighed, and if about 681grams 22 of salt and 170grams of lunar caustic be taken, then143grams of in-soluble silver chloride and 85grams of sodium nitrate will be obtained. The sum of the weights of the re-acting and resultant substances are seen to be similar and equal to 2281grams, which necessarily follows from the law of the indestructibility of matter.
|
|
Mendeleev's Periodic Principles |
See also: Development of the Periodic Table, de Chancourtois, Dobereiner, Mendeleev, Moseley, Newlands, Seaborg