 |
|
 |
Rusting of Metals
in Chapter 1 of Principles of Chemistry
Lavoisier proved by means of the balance that every case of rusting of metals or oxidation, or of combustion, is accompanied by an increase in weight at the expense of the atmosphere. He formed, therefore, the natural opinion that the heavier substance is more complex than the lighter one. Lavoisier's celebrated experiment, made in 1774, gave indubitable support to his opinion, which in many respects was contradictory to Stahl's doctrine. Lavoisier poured four ounces of pure mercury into a glass retort (fig. 3), whose neck was bent as shown in the drawing and dipped into the vessel x s, also full of mercury. The projecting end of the neck was covered with a glass bell-jar P. The weight of all the mercury taken, and the volume of air remaining in the apparatus, namely, that in the upper portion of the retort, and under the bell-jar, were determined before beginning the experiment. It was most important in this experiment to know the volume of air in order to learn what part it played in the oxidation of the mercury, 'because, according to Stahl, phlogiston is emitted into the air, whilst, according to Lavoisier, the mercury in oxidizing absorbs a portion of the air ; and consequently it was absolutely necessary to determine whether the amount of air increased or decreased in the oxidation of the metal. It was, therefore, most important to measure the volume scales , that is, it was oxidized or converted into an earth.
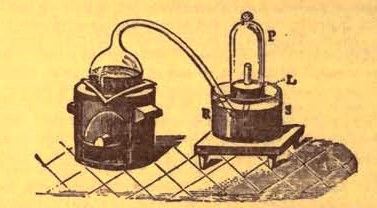
Fig. 3 Lavoisier's apparatus for determining the composition of air and the reason of metals increasing in weight when they are calcined in air.
This substance is the same mercury oxide which has already been mentioned. After the lapse of twelve days the apparatus was cooled, and it was then seen that the volume of the air in the apparatus had diminished during the time of the experiment. This result was in exact contradiction to Stahl's hypothesis. Out of 50 cubic inches of air originally taken, there only remained . Lavoisier's experiment led to other equally important results. The weight of the air taken decreased by as much as the weight of the mercury increased in oxidizing ; that is, the portion of the air was not destroyed, but only combined with mercury. This portion of the air may be again separated from the mercury oxide and has properties different from those of air. It is called 'oxygen.' That portion of the air which remained in the apparatus and did not combine with the mercury does not oxidize metals, and cannot support either combustion or respiration, so that a lighted taper is immediately extinguished if it be dipped into the gas which remains in the bell-jar. It is extinguished in the residual gas as if it had been plunged into water,' writes Lavoisier in his memoirs. This gas is called 'nitrogen.' Thus air is not a simple substance, but consists of two gases, oxygen and nitrogen, and therefore the opinion that air is an elementary substance is erroneous. The oxygen of the air is absorbed in combustion and the oxidation of metals, and the earths produced by the oxidation of metals are substances composed of oxygen and a metal. By mixing the oxygen with the nitrogen the same air as was originally taken is re-formed. It has also been shown by direct experiment that on reducing an oxide with carbon, the oxygen contained in the oxide is transferred to the carbon, and gives the same gas that is obtained by the combustion of carbon in air. Therefore this gas is a compound of carbon and oxygen, just as the earthy oxides are composed of metals and oxygen.
|
|
Mendeleev's Periodic Principles |
See also: Development of the Periodic Table, de Chancourtois, Dobereiner, Mendeleev, Moseley, Newlands, Seaborg