Module Eight of CCE 281 Corrosion: Impact, Principles, and Practical Solutions
Types of Water
Water is commonly described either in terms of its nature, usage, or origin. The implications in these descriptions range from being highly specific to so general as to be non-definitive. Ground waters originate in subterranean locations such as wells, while surface waters comprise the lakes, rivers, and seas.
Fresh Water
Fresh water may come from either a surface or ground source, and typically contains less than 1% sodium chloride. It may be either "hard" or "soft," i.e., either rich in calcium and magnesium saltsand thus possibly forming insoluble curds with ordinary soap. Actually, there are gradations of hardness, which can be estimated from the Langelier or Ryznarindexes or accurately determined by titration with standardized chelating agent solutions such as versenates.
Corrosion of waterworks is a major burden in many cities. Provide some examples from a search of the Internet.
Summarize the main elements that make natural fresh waters more or less corrosive.
Brackish Water
Brackish water contains between 1 and 2.5% sodium chloride, either from natural sources around otherwise fresh water or by dilution of seawater. Brackish water differs from open seawater in certain other respects. The biological activity, for example, can be significantly modified by higher concentrations of nutrients. Fouling is also likely to be more severe as a consequence of the greater availability of nutrients.
Within harbors, bays, and other estuaries, marked differences can exist in the amount and type of fouling agents present in the water. The main environmental factors responsible, singly or in combination, for these differences are the salinity, the degree of pollution, and the prevalence of silt. Moreover, the influence of these factors can be very specific to the type of organism involved. Apart from differences that can develop between different parts of the same estuary, there can also be differences between fouling in enclosed waters and on the open coast. In this respect the extent of offshore coastal fouling is strongly determined by the accessibility to a natural source of infection. Local currents, average temperature, seasonal effects, depth, and penetration of light are operative factors. The presence of pollutants can also be quite important and highly variable in coastal areas.
Seawater
Seawater typically contains about 3.5% sodium chloride, although the salinity
may be weakened in some areas by dilution with fresh water or concentrated by
solar evaporation in others. Seawater is normally more corrosive than fresh
water because of the higher conductivity and the penetrating power of the chloride
ion through surface films on a metal. The rate of corrosion is controlled by
the chloride content, oxygen availability, and the temperature. The 3.5% salt
contentof seawater produces the most corrosive chloride salt solution that can
be obtained as shown in the following Figure.
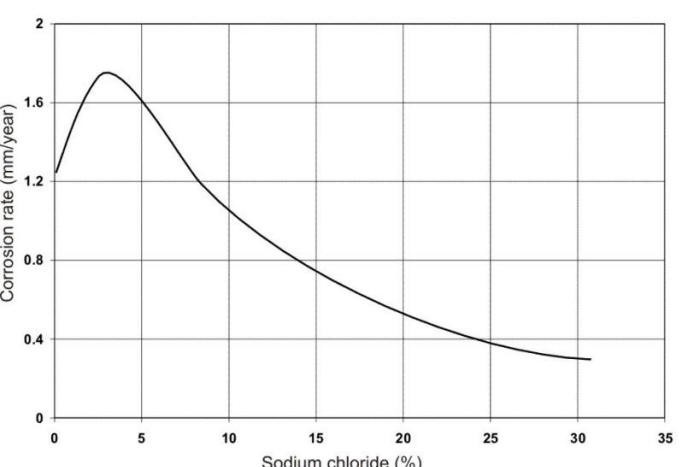
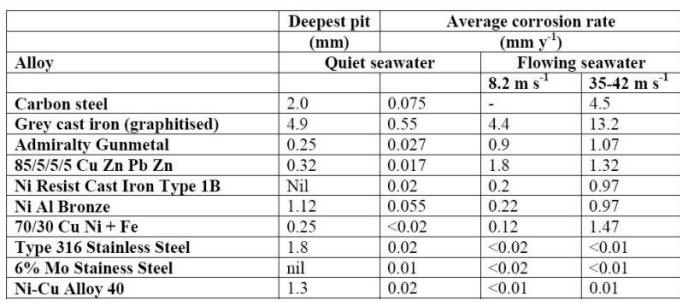
The combination of high conductivity and oxygen solubility is at a maximum at this point (oxygen solubility is reduced in more concentrated salt solutions). The corrosion of numerous metals in a wide range of saline waters is reported in the following Table.
Effect of velocity on the corrosion of metals in seawater
Propose an explanation for the maximum in corrosion rate reported in the corrosion rate vs % sodium chloride Figure.
Distilled or Demineralized Water
The total mineral content of water can be removed by either distillation or mixed-bed ion exchange. In the first case, purity is described qualitatively in some cases (e.g., triple-distilled water), but is best expressed, for both distilled and demineralized water, in terms of specific conductivity. Water also can be demineralized by reverse osmosis or electrodialysis.
Steam Condensate
Water condensed from industrial steam is called steam condensate. It approaches distilled water in purity, except for contamination (as by DO or carbon dioxide) and the effect of deliberate additives (e.g., neutralizing or filming amines).
Boiler Feedwater Make-up
The feedwater make-up for boilers is always softened and subsequently deaerated. It may vary in quality from fairly high dissolved solids (e.g., Zeolite-Treated), to very pure demineralized feed for high-pressure boilers. It tends to be highly corrosive, because of its softness, until thoroughly deaerated. This term is more precise than “boiler feedwater”, which may include recirculated steam condensate in various ratios to fresh make-up water.
Potable Water
Potable water is fresh water that is sanitized with oxidizing biocides such as chlorine or ozone to kill bacteria and make it safe for drinking purposes. By definition, certain mineral constituents are also restricted. For example, the chlorinity will be not more than 250 ppm chlorideion in the United States or 400 ppm on an international basis.
Process or Hydrotest Water/Firewater
These terms are essentially non-definitive, since the water employed may be of almost any chemistry, ranging from demineralized water to quite saline fresh water or even seawater in some cases. “Produced water” is that which originates in oil and gas production, emanating from geological sources with the hydrocarbons.
Cooling Water
Cooling water is another undefined term, although it implies that any necessary treatment against excessive scaling or corrosion has been applied, or corrosion-resistant material selected. This may include anything from fresh water to seawater, and may comprise either an open- or closed-loop system, or a once-through system.
Waste Water
By definition, waste water is any water that is discarded after use. Sanitary waste from private or industrial applications is contaminated with fecal matter, soaps, detergents, etc., but is quite readily handled from a corrosion standpoint. Industrial wastes from chemical or petrochemical sources can contain strange and specific contaminants which greatly complicate materials selection, especially in the uses of plastics and elastomers.
| (previous) | Page 3 of 4 | (next) |

Connect with us
Contact us today