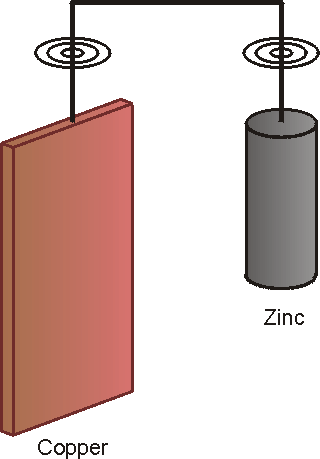
The following graphic shows cathodic protection of copper with a zinc anode submerged in water and the subsequent graphic illustrates the electrical equivalent circuit of this arrangement.
In the large galvanic cell so formed, the zinc cylinder corroded away in a manner that can be explained using the following electrical diagram:
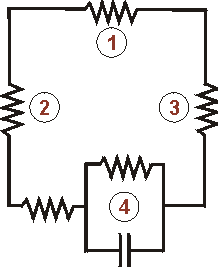
Represents the electrical resistance of the wire connecting the two metal, one is the anode being sacrificed and the other the metal being protected
.. the electrical resistance through the copper plate
.. the electrical resistance through the zinc cylinder
.. the complex equivalent circuit for the metallic interfaces
An anode is, by definition, the corroding member in a dissimilar metal combination. A galvanic or sacrificial anode can be described as a metal which will develop a negative voltage with respect to the corroding structure and will discharge current that will flow through the environment to the structure.
This is not as complicated as it seems. A voltage will develop between the corroding structure and the anode. The structure will therefore become positive (+) with respect to the anode. There are certain requirements for a metal to be a practical galvanic anode material:
The potential between the anode and corroding structure must be large enough to overcome the anode-cathode cells on the corroding structure
The anode material must have sufficient electrical energy and current to permit reasonably long life with a practical amount of anode material
Anodes must have good efficiency, meaning that a high percentage of the electrical energy content of the anode should be available for useful CP current output. The balance of the energy that is consumed in self-corrosion of he anode itself should be very small
See also: Conductivity cell, Corrosion cell definition, Daniell cell, Electrochemistry dictionary, Impressed current cathodic protection system, Natural corrosion cells, Rotating cylinder test cell, Sacrificial anode cathodic protection