
 |
The following Figure illustrates the principle of a Daniell cell in which copper and zinc metals are immersed in solutions of their respective sulfates.
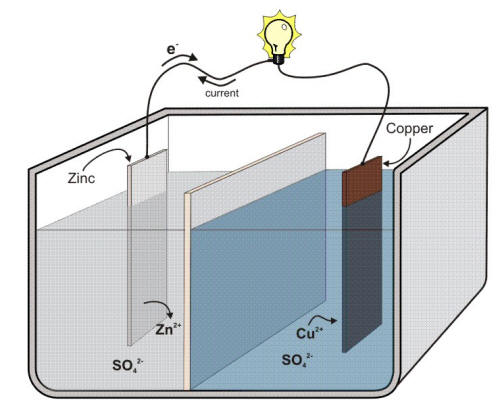
Schematic of a Daniell cell.
The Daniell cell was the first truly practical and reliable electric battery that supported many nineteenth century electrical innovations such as the telegraph. In the process of the reaction, electrons can be transferred from the corroding zinc to the copper through an electrically conducting path as a useful electric current. Zinc more readily loses electrons than copper, so placing zinc and copper metal in solutions of their salts can cause electrons to flow through an external wire which leads from the zinc to the copper.
![]()
![]()
The difference in the susceptibility of two metals to corrode can often cause a situation that is called galvanic corrosion named after Luigi Galvani, the discoverer of the effect. The purpose of the separator shown in the previous Figure is to keep each metal in contact with its own soluble sulfates, a technical point that is critical in order to keep the voltage of a Daniell cell relatively constant. The same goal can be achieved by using a salt bridge between two different beakers as shown in the following Figure.
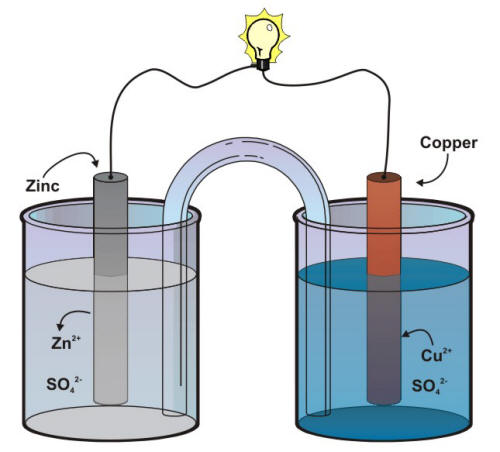
Schematic of a Daniell cell with a salt bridge
The salt bridge, in that case, provides the electrolytic path that is necessary to complete an electrochemical cell circuit. This situation is common in natural corrosion cells where the environment serves as the electrolyte that completes the corrosion cell. The conductivity of an aqueous environment such as soils, concrete, or naturals waters has often been related to its corrosivity.
![]()
Conc1 and Conc2 in equation describe respectively the concentration of zinc sulfate and copper sulfate that may differ in the two half-cells while the two slanted bars (//) describe the presence of a separator. The same equation also identifies the zinc electrode as the anode that is negative in the case of a spontaneous reaction and the copper cathode as positive.
Why is a separator commonly used between the anodic and cathodic half cells of a Daniell cell?
Elaborate on the effect the absence of a separator would have on the potential generated by a Daniell cell. Make reference to the Nernst equation described in Module 4 to support your arguments.
Write a short-hand description of the reactions involved in the corrosion of zinc.
| (previous) | Page 2 of 6 | (next) |