
 |
The saturation of water refers to the solubility product (Ksp) of a compound. For example, the ion activity product (IAP) of the ions involved when calcium carbonate is the scalant is:
![]()
The saturation level (SL) of water is defined as ratio of the ion activity product over Ksp as in the following:
![]()
In this example, water is said to be saturated with calcium carbonate when it will neither dissolve, or precipitate calcium carbonate scale. This equilibrium condition is based upon an undisturbed water, at constant temperature, which is allowed to remain undisturbed for an infinite period of time.
Water is said to be undersaturated if it can still dissolve calcium carbonate. Supersaturated water will precipitate calcium carbonate from water if allowed to rest. If water is undersaturated with respect to calcium carbonate, the SL value will be less than 1.0. When water is at equilibrium, SL will be 1.0 by definition. Water which is supersaturated with calcium carbonate will have a saturation level greater than 1.0. As the saturation level increases beyond 1.0, the driving force for calcium carbonate crystal formation or crystal growth increases.
The SL definition can be simplified if the activity coefficients are incorporated into the solubility product in order to use a more practical concentration unit. The conditional solubility product (Kspc) incorporates the activity coefficients into the solubility product in which concentrations are expressed as molarities ([ ]): (reference)
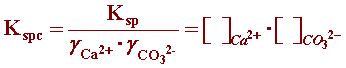
See also: Calcium carbonate, Carbon dioxide, Chlorination, Dissolved oxygen, Langelier calculation, Langelier index, Larson-Skold index, Oddo-Tomson index, pH, Puckorius index, Ryznar index, Scaling Indices, Stiff-Davis index, Total dissolved solids, Water corrosivity,
Click here to read what some experts have to say on this topic more on this topic.